Carbohydrate Fermentation Test
- The carbohydrate fermentation test is a microbiological diagnostic method used to assess whether bacteria have the capability to ferment specific carbohydrates, leading to the production of acids and/or gases. This test is pivotal in distinguishing between different types of bacteria based on their metabolic properties.
- Carbohydrates are fundamental organic compounds composed of carbon, hydrogen, and oxygen, typically in a ratio represented by the formula (CH2O)n. These substances are classified into three main types based on their molecular structure and size: monosaccharides, which are simple sugars with three to seven carbon atoms; disaccharides, which consist of two monosaccharide units linked by a glycosidic bond; and polysaccharides, which are complex molecules made up of eight or more monosaccharide units.
- During carbohydrate fermentation, microorganisms such as bacteria, fungi, and yeasts break down these sugars. This catabolic process involves the decomposition of complex organic molecules into simpler forms, releasing energy that the organisms can use.
- The specific pathways and enzymes involved in carbohydrate breakdown vary among different species, making this a critical factor in their identification and classification.
- Fermentation characteristics are often unique to particular genera or species of microorganisms. Therefore, the carbohydrate fermentation test is extensively used in microbiology to help differentiate and identify various microbial species based on their fermentative capabilities.
- This test not only sheds light on the metabolic functions of the microorganisms but also assists in understanding their ecological roles and potential impacts in medical, environmental, and industrial contexts.
Purpose of Carbohydrate fermentation test
The carbohydrate fermentation test serves several important purposes in microbiology, primarily related to identifying and understanding the metabolic capabilities of various microorganisms. Here are the key objectives of conducting this test:
- Assessment of Fermentation Ability:
- Determine if a microorganism can ferment specific carbohydrates.
- Establish the ability to convert these carbohydrates into energy.
- Identification of Metabolic Products:
- Detect the production of organic acids as end products of fermentation.
- Test for the release of gases, which can be indicative of anaerobic fermentation processes.
- Microbial Classification:
- Utilize differences in fermentation capabilities to classify and differentiate between species and strains of bacteria, yeasts, and fungi.
- Provide a basis for phenotypic characterization in microbial taxonomy.
- Diagnostic Tool:
- Use variations in fermentation patterns to identify pathogenic organisms in clinical samples.
- Aid in diagnosing infections based on the metabolic properties of the causative microorganisms.
Carbohydrate fermentation test Principle
Carbohydrate fermentation is the method in which microorganisms utilize carbohydrates to create energy through the production of ATP which is the primary energy source for the organism. After entering a cell, glucose can be catabolized in an aerobic manner (in with the help of O2) and in which molecular oxygen is an electron acceptor (oxidative pathway) as well as the other way (in the absence of O2) where organic ions may function as an electron acceptor (fermentative pathway). The metabolic end-products of the carbohydrate fermentation could include organic acids (lactic acid, formic, and acetic acids) or organic acids and gases (hydrogen as well as carbon dioxide).
The degradation of carbohydrates (monosaccharide disaccharide as well as polysaccharide) by microorganisms during the anaerobic environment is performed in the fermentation tube made up of Durham tube to detect of gas production. A fermentation medium is made up of a base medium that contains one specific carbohydrate (glucose sucrose, sucrose, or cellulose) and an indicator for pH (phenol red, the indicator of Andrade bromocresol, or Andrade’s indicator).
As the organism ferments sugars, acid organic compounds (Lactic acid or formic acid as well as acetic acids) are produced, which change the medium yellow and a decrease in acidity (acidic-below the pH value of 6.8). The alteration in the pH indicator of the fermentation tube and in the gas production within the Durham tube are a sign of the metabolic reaction that results in the production of acid end products and gas.
The change in color only happens and is evident when an adequate amount of acid is produced, since bacteria can utilize peptone for creating alkaline products. The breakdown of peptones in the broth can lead to the production of alkaline ending products, which change the color of the broth, which is pink frequently on the highest point in the tube.
Media for Carbohydrate fermentation test
The media used for carbohydrate fermentation tests are crucial for diagnosing and understanding the metabolic characteristics of microorganisms. Here’s an overview of the typical media formulations used in these tests, specifically focusing on variations using phenol red as the pH indicator:
- Common Types of Media:
- Phenol Red Glucose Broth: Used to test for the fermentation of glucose.
- Phenol Red Lactose Broth: Utilized for assessing lactose fermentation.
- Phenol Red Maltose Broth: Applied to evaluate maltose fermentation.
- Phenol Red Mannitol Broth: Used for determining mannitol fermentation capabilities.
- Phenol Red Sucrose Broth: Employed to test sucrose fermentation.
- Preparation and Composition:
- Commercial Availability: These media can be purchased ready-made from commercial suppliers, ensuring consistency and reliability in test results.
- DIY Preparation: Alternatively, laboratories can prepare their own media by sourcing a base of phenol red broth and adding specific carbohydrates based on the requirements of the tests they intend to conduct.
- Detailed Composition of Phenol Red Carbohydrate Broth:
- Proteins: Trypticase or protease peptone No. 3, typically around 10 grams, serves as the protein source.
- Salt: Sodium chloride, generally about 5 grams, is added to maintain the osmotic balance.
- Nutrient Supplement: Beef extract, although optional, can be included at 1 gram to enhance nutrient content.
- pH Indicator: Phenol red is used, with about 7.2 ml of a 0.25% solution, to visually indicate changes in pH due to fermentation.
- Carbohydrate Source: Approximately 10 grams of the specific carbohydrate intended for testing.
Composition of Phenol Red Carbohydrate Broth
| Ingredients | Amount |
| Trypticase or protease peptone No. 3 | 10 g |
| Sodium chloride (NaCl) | 5 g |
| Beef extract (optional) | 1 g |
| Phenol red (7.2 ml of 0.25% phenol red solution) | 0.018 g |
| Carbohydrate source | 10g |
Carbohydrate Fermentation Test Procedure
Preparation of the media
- Ingredient Mixing:
- Combine all required ingredients in 1000 mL of deionized or distilled water. Ensure that you choose only one carbohydrate source based on the specific test you are conducting.
- Dissolution of Ingredients:
- Heat the mixture gently to help dissolve all components thoroughly. Avoid boiling to prevent degradation of heat-sensitive ingredients.
- Distribution into Test Tubes:
- Dispense approximately 4-5 mL of the phenol-red carbohydrate broth into each 13x100mm test tube. This volume ensures enough space for any gas production to be clearly observed.
- Placement of Durham Tubes:
- Insert a small, inverted Durham tube into each test tube before autoclaving. The Durham tube is crucial for capturing any gases produced during fermentation, providing a visual indication of gas production.
- Sterilization:
- Autoclave the prepared media at 120°C for 15 minutes. This sterilization process not only eliminates potential contaminants but also forces the broth to fill the inverted Durham tube, ensuring it is ready to capture gases effectively.
- Note: For carbohydrates like lactose, arabinose, salicin, maltose, sucrose, and xylose, reduce the autoclaving time to just 3 minutes at 121°C. These sugars are more susceptible to decomposition under prolonged high-temperature exposure, which could affect test results.
Note: The broth will have light red in color, and the pH of the final product will be 7.4 + 0.2.
If you prefer, make the base of phenol red broth that you heat sterilize and cool to 45 degrees Celsius. Separately prepare a specific carbohydrate mixture then remove the solution using membrane filters (pore dimension: 0.45 mm). Add the solution of carbohydrate in the broth base, and then mix. The ideal carbohydrate content is 1 %.
Inoculation and Incubation into the fermentation medium
- Inoculation of Test Tubes:
- Utilize a sterile inoculating needle or loop to introduce the microorganism into each test tube containing the fermentation medium. Ensure the tool is sterilized between each inoculation to prevent cross-contamination.
- Alternatively, inject 2 to 3 drops of a culture grown in brain-heart infusion broth for 18-24 hours directly into each test tube. This method ensures a consistent and active culture is introduced.
- Incubation Conditions:
- Place the inoculated tubes in an incubator set at 35 to 37 degrees Celsius.
- Incubate the cultures for 18-24 hours, which is generally sufficient for most organisms to exhibit fermentative activity.
- Extended Incubation for Negative Tests:
- If initial observations do not show signs of fermentation (i.e., no gas or acid production), consider extending the incubation period. Some organisms require more time to metabolize the carbohydrate and produce detectable levels of acid or gas.
- Observation and Examination:
- After incubation, examine each tube for evidence of fermentation. Look for:
- Gas Production: Check the Durham tube for any gas bubbles, which indicate fermentative gas production.
- Acid Production: Observe any color change in the medium due to the pH indicator. A yellow color typically indicates acid production, reflecting a drop in pH.
- After incubation, examine each tube for evidence of fermentation. Look for:
Result and Interpretation of Carbohydrate fermentation test
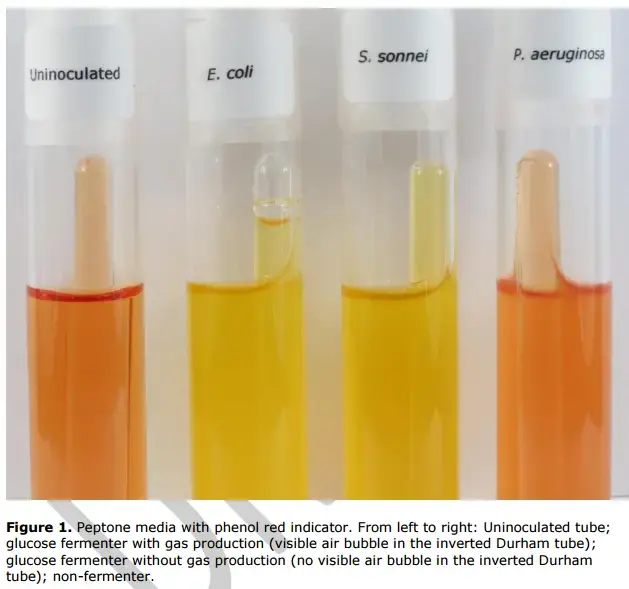
- Acid Production:
- Positive Result: If the media in the tube turns yellow after incubation, this indicates a decrease in pH. Such a color change, due to the phenol red indicator, signifies acid production from the fermentation of the carbohydrate present in the medium.
- Negative Result: A test tube that retains the red color of the medium suggests that the bacterium cannot ferment the specific carbohydrate provided, implying a lack of acid production.
- Gas Production:
- Positive Result: The presence of a bubble, regardless of size, within the inverted Durham tube is a clear sign of gas production. This indicates that the bacteria produce gas as a byproduct of fermenting the given carbohydrate.
- Negative Result: Absence of bubbles in the Durham tube means no gas has been produced during fermentation, classifying the bacteria as anaerogenic with respect to the carbohydrate tested.
Uses of Carbohydrate Fermentation Test
- Differentiation of Bacterial Species:
- Utilize fermentation patterns to distinguish between different species or groups of bacteria based on their ability to ferment specific carbohydrates.
- Identification within Enterobacteriaceae Family:
- Confirm that members of the Enterobacteriaceae family can ferment glucose, often doing so anaerobically.
- Discrimination among Proteus Species:
- Use maltose fermentation to differentiate Proteus vulgaris (which ferments maltose) from Proteus mirabilis (which does not ferment maltose).
- Distinction between Neisseria Species:
- Identify Neisseria gonorrhoeae and Neisseria meningitidis by their ability to ferment glucose; however, only Neisseria meningitidis can ferment maltose.
- Rapid Identification Tests:
- Employ Rapid Carbohydrate Utilization Tests (RCUTs) to identify Corynebacterium diphtheriae and differentiate it from other Corynebacterium species.
Precautions of Carbohydrate Fermentation Test
- Loop Sterilization:
- After inoculating the medium with a specific sugar, sterilize the inoculating loop thoroughly to avoid cross-contamination with other carbohydrates or media.
- Durham Tube Inspection:
- Do not use fermentation tubes if the Durham tubes inside them are only partially filled with medium or if they already contain bubbles prior to inoculation, as this could lead to erroneous interpretations of gas production.
- Incubation Duration:
- Avoid over-incubation. Prolonged incubation may lead to the degradation of proteins within the medium, potentially resulting in false-positive outcomes for sugar fermentation.
Limitations of Carbohydrate Fermentation Test
- Timing of Readings:
- Accurate readings are typically obtained within 24 hours. Delays in measurement beyond this period may not reflect true fermentation capabilities if acid production has not occurred.
- Peptone Metabolism:
- An absence of color change, or an alkaline result, may arise if the organism metabolizes peptone rather than the carbohydrate. This peptone breakdown can mask the actual fermentation process, leading to misinterpretation of results.
- Nonspecific Gas Production:
- The presence of gas in Durham tubes doesn’t always confirm fermentation of the carbohydrate being tested, as some bacteria produce gas through protein metabolism.
- Slow Fermenters:
- Some organisms may ferment carbohydrates slowly, requiring extended incubation times that could complicate the interpretation of results.
- Neutralization of Acids:
- Organisms that produce both acid and alkaline products may neutralize their environment, leading to no change in the pH indicator despite the occurrence of fermentation.
- Overcrowding of Media:
- High bacterial loads can lead to the rapid exhaustion of carbohydrates, subsequent utilization of peptones, and an alkaline shift, which might erroneously suggest a negative fermentation result.
- Indicator Exhaustion:
- The pH indicator’s color change is dependent on the concentration of acidic or alkaline products; extensive fermentation may exhaust the indicator, reverting the color change and leading to false interpretations.
- Inhibition by Byproducts:
- Acidic byproducts of fermentation may inhibit the growth of some bacteria, preventing them from fully demonstrating their fermentative potential within the standard incubation period.
- Ambiguity in Weak Fermentation:
- Weak fermenters may produce such a slight change in pH that it is challenging to distinguish between a true positive and a negative result.
- Requirement for Pure Cultures:
- The test requires a pure culture for accurate results; contaminants can ferment the carbohydrate, leading to a false-positive result for the organism of interest.
References
- Tille P.M (2014)Bailey and Scott’s diagnostic microbiology, Thirteen editions, Mosby, Inc., an affiliate of Elsevier Inc., 3251 Riverport Lane, St. Louis, Missouri 63043
- Aneja K.R. 2003. Experiments in Microbiology, Plant Pathology, and Biotechnology, fourth revised edition, New Age International (P) limited, Ansari road, Daryaganj, New Delhi-110002.
FAQ
What is the purpose of phenol red in the carbohydrate fermentation test?
Phenol red is used as a pH indicator in the carbohydrate fermentation test. Its primary purpose is to signal a change in pH that occurs as a result of fermentation. Here’s how it works:
Indicator of Acid Production: When bacteria ferment the carbohydrate present in the medium, they produce acid. Phenol red changes color from red to yellow under acidic conditions, indicating a drop in pH and thus confirming acid production.
Detection of Negative Results: If the pH remains neutral or becomes alkaline (due to peptone metabolism by some bacteria), phenol red stays red or may even turn pink, signaling that no acid has been produced from fermentation of the carbohydrate.
Phenol red is an essential component because it provides a simple and immediate visual cue to interpret the results of the test.
What type of media is used for carbohydrate fermentation test?
The media used for carbohydrate fermentation tests are liquid broth media that typically contain:
Basic Nutrients: These include sources of nitrogen and vitamins, such as peptones and beef extract, which support the growth of a wide variety of bacteria.
A Single Carbohydrate: A specific type of sugar (e.g., glucose, lactose, sucrose, mannitol) is added to the medium to assess if the bacterium can ferment that particular carbohydrate.
A pH Indicator: A compound such as phenol red is included in the medium. Phenol red turns yellow under acidic conditions, which indicates the production of acid from carbohydrate fermentation, and remains red or turns pink if the medium is neutral or alkaline.
A Gas Detection System: An inverted Durham tube is often placed inside the test tube to capture any gas produced as a byproduct of fermentation.
What is the purpose of carbohydrate fermentation test?
The carbohydrate fermentation test serves several important purposes in microbiology:
Identification of Microorganisms: Different bacteria have distinct patterns of carbohydrate fermentation, which can be used to help identify and differentiate them at the species or even strain level.
Diagnosis of Infections: In clinical microbiology, the ability of bacteria to ferment specific carbohydrates can be a key factor in diagnosing infections caused by certain pathogens.
Understanding Metabolic Capabilities: The test provides insights into the metabolic processes of bacteria, showing whether they can utilize a particular carbohydrate as an energy source and what end products they generate.
Characterization of Bacteria: It helps in the phenotypic characterization of bacteria, which is useful for taxonomic classification in research settings.
Food and Beverage Industry: In the food and beverage industry, it can be used to assess the fermentation capabilities of yeasts and bacteria important in the production of fermented foods and drinks.
Biotechnology Applications: The test can be used in biotechnology to select for or engineer strains that have desired fermentation characteristics for various applications, including biofuel production.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.