Xylose Lysine Deoxycholate (XLD) agar is a selective, differential, and indicator medium used in microbiology for the isolation and identification of enteric pathogens. It contains various components that contribute to its selectivity and differentiation capabilities.
The medium employs sodium deoxycholate, a bile salt, as a selective agent to inhibit the growth of gram-positive organisms, making it specific for gram-negative bacteria.
Differentiation is achieved through three indicator systems present in XLD agar:
- Fermentable Carbohydrates: Xylose, lactose, and sucrose are included in different concentrations as fermentable carbohydrates. Organisms capable of fermenting these sugars produce acid, resulting in a decrease in pH. This acidic pH is indicated by a color change from red to yellow.
- Lysine Decarboxylation: In the absence of lactose and sucrose fermentation, the decarboxylation of lysine by certain organisms leads to an increase in pH, returning the medium to an alkaline state. This alkaline pH causes the medium to revert back to a red color.
- Hydrogen Sulfide (H2S) Indicator: Sodium thiosulfate and ferric ammonium citrate serve as indicators of H2S production under alkaline conditions. H2S-producing organisms create colonies with black centers due to the reaction between hydrogen sulfide and ferric ions.
The interpretation of XLD agar results is as follows:
- Organisms incapable of utilizing the carbohydrates, such as Shigella and Edwardsiella, do not produce significant changes, resulting in red colonies and a red medium after incubation.
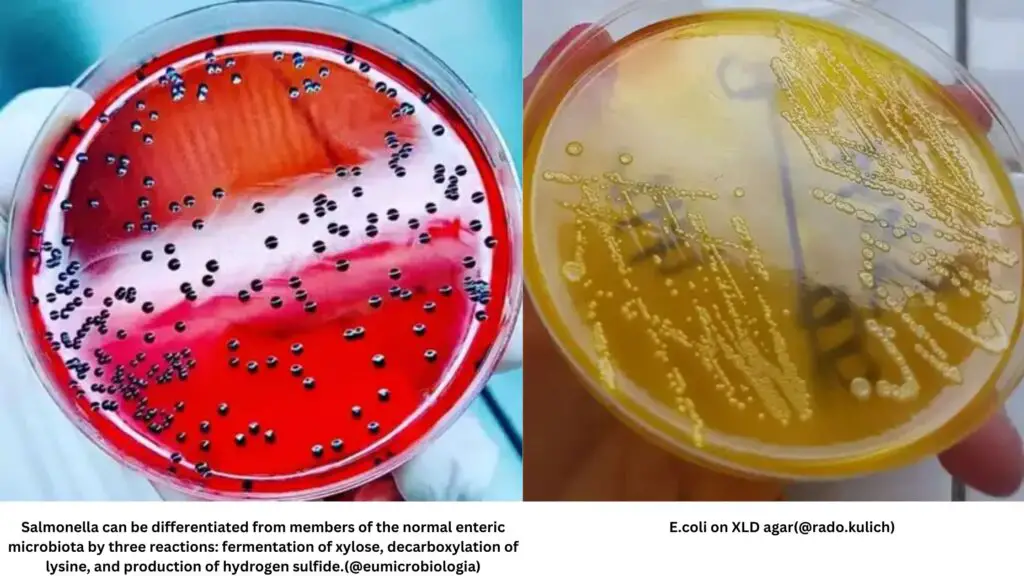
- Organisms capable of fermenting xylose only, such as Salmonella, deplete the xylose and switch to lysine fermentation, leading to an alkaline pH and the return of a red color. The presence of Salmonella and Edwardsiella spp. can be differentiated from Shigella by their ability to produce hydrogen sulfide.
- Other uninhibited organisms capable of fermenting lactose or sucrose continue to produce acid, resulting in yellow colonies due to the persistent acidic pH. These organisms include Escherichia, Klebsiella, Serratia, Citrobacter koseri, and Yersinia enterocolitica.
- Lysine-negative organisms, such as Proteus species, also produce yellow colonies on XLD agar.
The inclusion of an H2S indicator system enhances the differentiation capacity of XLD agar, allowing for the visualization of hydrogen sulfide production and the formation of colonies with black centers.
Overall, XLD agar is a valuable medium for the selective isolation and differentiation of enteric pathogens based on their carbohydrate fermentation patterns, lysine decarboxylation, and hydrogen sulfide production.
What is Xylose Lysine Deoxycholate (XLD) Agar?
- Xylose Lysine Deoxycholate (XLD) Agar is a specialized growth medium used for the isolation and differentiation of enteric gram-negative pathogens, particularly Salmonella and Shigella, from various sources such as clinical samples, environmental samples, and food samples. It is a moderately selective and differential medium that aids in the identification of these pathogens.
- The composition of XLD Agar includes several key ingredients that contribute to its selective and differential properties. It contains yeast extract, which provides essential nutrients like nitrogen and vitamins for bacterial growth. The sugars xylose, lactose, and sucrose are also included in the medium as fermentable carbohydrates. Among these sugars, xylose is mainly incorporated because Shigella species cannot ferment it, while most other enteric bacteria can. This characteristic helps differentiate Shigella from other enterics.
- Additionally, sodium chloride is included in XLD Agar to maintain the osmotic balance of the medium. Lysine, an amino acid, is added to differentiate the Salmonella group from non-pathogenic bacteria. Salmonella bacteria rapidly ferment xylose, depleting its supply. Subsequently, lysine is decarboxylated by the enzyme lysine decarboxylase, leading to the formation of amines and an alkaline pH that mimics the reaction observed in Shigella. To prevent this reaction by lysine-positive coliforms, lactose and sucrose are added, which produce excess acid and cause a color change in the pH indicator, phenol red, from red to yellow.
- The XLD Agar formulation includes an indicator system to detect the production of hydrogen sulfide (H2S). This system consists of sodium thiosulfate and ferric ammonium citrate. Salmonella species metabolize thiosulfate to produce hydrogen sulfide, resulting in the formation of colonies with black centers. Non-pathogenic bacteria that produce H2S but do not decarboxylate lysine prevent blackening of the medium due to the acid reaction they produce.
- XLD Agar serves as both a selective and differential medium. It selectively inhibits the growth of gram-positive microorganisms due to the presence of sodium deoxycholate, while allowing the growth of gram-negative enteric bacteria. However, it should be noted that some Proteus strains may give false positive reactions by developing red to yellow coloration, with black centers similar to Salmonella. Non-enteric bacteria like Pseudomonas and Providencia may exhibit red colonies on XLD Agar.
- The medium was developed by Taylor for the isolation and differentiation of enteric pathogens, particularly Salmonella Typhi, from other Salmonella species. It has been recommended by the American Pharmacopoeia (USP) and European Pharmacopoeia (EP) for the enumeration of microorganisms. XLD Agar exhibits increased selectivity and sensitivity compared to other plating media such as SS Agar, EMB Agar, and Bismuth Sulphite Agar.
- In summary, Xylose Lysine Deoxycholate (XLD) Agar is a selective and differential growth medium used for the isolation and differentiation of Salmonella and Shigella species. Its composition allows for the detection of xylose fermentation, lysine decarboxylation, and hydrogen sulfide production, which are key characteristics used in the identification of these enteric pathogens.
Composition of Xylose Lysine Deoxycholate (XLD) Agar
| Ingredients | Gms/Litre |
| Yeast extract | 3.0 |
| L- Lysine | 5.0 |
| Lactose | 7.5 |
| Sucrose | 7.5 |
| Xylose | 3.5 |
| Sodium chloride | 5.0 |
| Sodium deoxycholate | 2.5 |
| Sodium thiosulphate | 6.8 |
| Ferric ammonium citrate | 0.8 |
| Phenol red | 0.08 |
| Agar | 15.0 |
Final pH (at 25°C) 7.4±0.2
Principle of Xylose Lysine Deoxycholate (XLD) Agar
The principle of Xylose Lysine Deoxycholate (XLD) Agar lies in its selective and differential properties for the isolation and differentiation of enteric gram-negative pathogens, particularly Salmonella and Shigella.
The medium contains yeast extract, which provides essential nitrogen and vitamins necessary for bacterial growth. It also utilizes sodium deoxycholate as a selective agent, making it inhibitory to gram-positive microorganisms. This selectivity helps in isolating the target gram-negative pathogens.
XLD Agar incorporates sugars such as xylose, lactose, and sucrose as sources of fermentable carbohydrates. However, it mainly includes xylose because Shigella species cannot ferment xylose, while most other enteric bacteria can. This allows for the differentiation of Shigella from other enterics.
Sodium chloride is included in the medium to maintain the osmotic balance necessary for bacterial growth. Lysine, an amino acid, is added to differentiate the Salmonella group from non-pathogens. Salmonella bacteria rapidly ferment xylose and exhaust its supply. Following this, lysine is decarboxylated by the enzyme lysine decarboxylase, leading to the formation of amines and a subsequent increase in alkaline pH, mimicking the reaction observed in Shigella. However, to prevent this reaction by lysine-positive coliforms, lactose and sucrose are added, which produce excess acid. The degradation of xylose, lactose, and sucrose to acid causes the pH indicator, phenol red, to change its color to yellow.
Bacteria that decarboxylate lysine to cadaverine can be identified by the appearance of a red coloration around the colonies due to an increase in pH. These reactions can occur simultaneously or successively, resulting in various shades of color or a change in color from yellow to red upon prolonged incubation.
To enhance the differentiating ability of the medium, an indicator system for hydrogen sulfide (H2S) is included. This system consists of sodium thiosulfate and ferric ammonium citrate. Salmonella species metabolize thiosulfate to produce hydrogen sulfide, which leads to the formation of colonies with black centers. Non-pathogenic H2S producers do not decarboxylate lysine, and their acid reaction prevents the blackening of the colonies.
In summary, Xylose Lysine Deoxycholate (XLD) Agar selectively inhibits gram-positive microorganisms and differentiates Salmonella and Shigella based on their ability to ferment xylose, decarboxylate lysine, and produce hydrogen sulfide. The medium’s composition and indicator systems provide visual cues, such as color changes and colony appearance, to aid in the identification and differentiation of these enteric pathogens.
Type of specimen used in Xylose Lysine Deoxycholate (XLD) Agar
Samples from clinical trials such as
- Blood
- Faeces
- Food and dairy samples
- Water samples.
Purpose of Xylose Lysine Deoxycholate (XLD) Agar
This agar is recommended for the isolation and identification of Salmonella Typhi and other Salmonella species using clinical and nonclinical samples.
Preparation of Xylose Lysine Deoxycholate (XLD) Agar
To prepare Xylose Lysine Deoxycholate (XLD) Agar, follow the steps below:
- Suspend 56.68 grams of XLD Agar powder in 1000 ml of distilled water. Use a container or flask that is suitable for heating.
- Heat the mixture with frequent agitation until it reaches the boiling point. Ensure that the agar is completely dissolved. It is important not to autoclave or overheat the medium, as this can lead to undesirable changes in its composition.
- Immediately transfer the agar mixture to a water bath set at a temperature of 50°C. This helps the medium cool down gradually and prevents the formation of precipitates.
- Once the medium has cooled to an appropriate temperature, pour it into sterile Petri plates. The volume of agar poured into each plate should be sufficient to provide a suitable depth for bacterial growth.
- It is advisable to avoid preparing large volumes of XLD Agar that would require prolonged heating. Prolonged heating can result in the formation of precipitates, which can affect the quality and performance of the medium.
- Allow the poured plates to solidify at room temperature or in a controlled environment. Once solidified, the plates are ready to be used for bacterial culture and analysis.
It is important to maintain aseptic conditions throughout the preparation process to prevent contamination. Proper labeling of the plates with relevant information such as date and batch number is also recommended for proper identification and traceability.
Physical properties of Xylose Lysine Deoxycholate (XLD) Agar
- Appearance: Light yellow to pink homogeneous free flowing powder.
- pH: 7.4 +0.2
- Gelling: Firm, comparable with 1.5% Agar gel
- Colour and Clarity of prepared medium: Red coloured clear to slightly opalescent gel forms in Petri plates
- Reaction: Reaction of 5.67% w/v aqueous solution at 25°C . pH : 7.4±0.2
Cultural Response: After incubation at 35-37degC, and at a specified amount of time the cultural response was observed. For bacteria growth on Soyabean Casein Digest Agar, the recovery rate is 100%.
Technique on XLD Agar
You can either plate the feces directly or use selective enrichment broths before streaking out. Salmonella enrichment can be done with Tetrathionate Broth (CM0029) or Selenite Broth (CM0395).
- Innoculate the dried, poured plates with a loopful inoculum, either from stool samples, rectal swabs, or a suitable enrichment broth.
- For 18-24 hours, incubate the plates at 35-37degC.
- Look out for common colonies.
Refer to the appropriate standards for testing food samples
Results and Colony Characteristics of Xylose Lysine Deoxycholate (XLD) Agar
Result interpretation on XLD Agar is based on the characteristics of the colonies that develop after incubation with the tested organisms. The different colony colors and characteristics help in the identification of potential pathogens. The following interpretations can be made:
- Red Colonies: Red colonies indicate an alkaline reaction, which can be due to the non-fermentation of xylose, lactose, and sucrose by the tested organism. It can also be the result of xylose fermentation followed by lysine decarboxylation. Possible pathogens associated with red colonies include Shigella spp, Providencia spp, Pseudomonas spp, and H2S non-producing Salmonella spp.
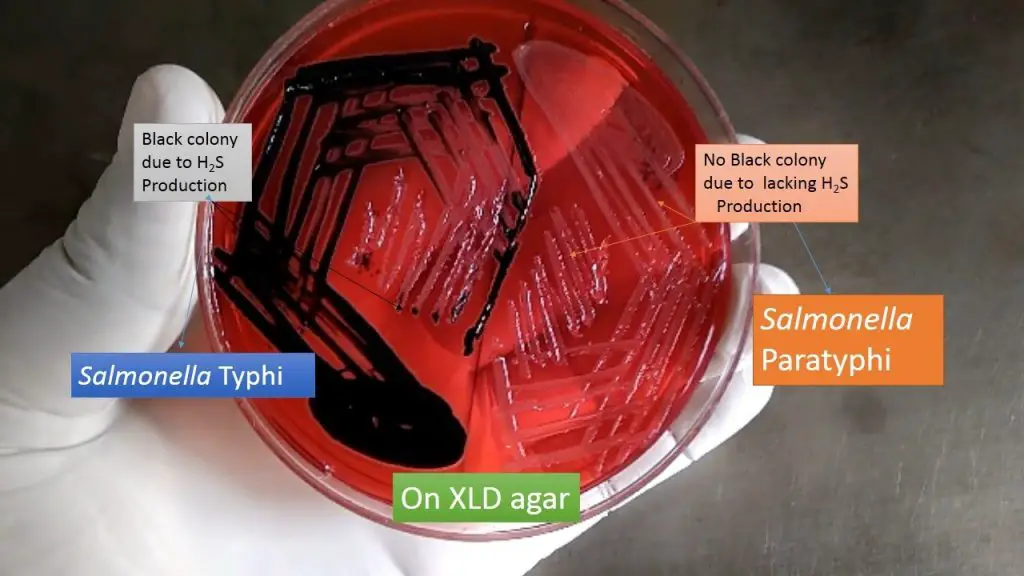
- Red Colonies with a Black Center: These colonies indicate positive reactions for xylose fermentation, lysine decarboxylase activity, and hydrogen sulfide production. The black center is a result of the formation of hydrogen sulfide in an alkaline pH. This pattern is commonly observed in H2S-producing Salmonella spp, such as S. Typhi and S. Typhimurium.
- Yellow Opaque Colonies: Yellow opaque colonies signify the fermentation of xylose while not fermenting lactose and sucrose. These colonies are lysine-negative and produce an acidic pH. Possible pathogens associated with this pattern include E. coli, Klebsiella/Enterobacter, Citrobacter, Serratia, and Proteus spp.
- Yellow Colonies: Yellow colonies indicate the fermentation of lactose or sucrose by the tested organism. These colonies are lysine-negative and produce an acidic pH. They are commonly observed in coliforms and sucrose-positive Proteus spp.
By observing and interpreting the colony characteristics and color reactions on XLD Agar, it is possible to narrow down the potential pathogens present in the tested sample. However, it is important to note that further confirmatory tests, such as biochemical, immunological, molecular, or mass spectrometry testing, are recommended for complete identification and accurate characterization of the isolated organisms.
| Colony characteristics on XLD Agar | Basis of reaction | Possible pathogens |
| Red colonies | Alkaline reaction, non-fermentation of xylose/lactose/sucrose or fermentation of xylose followed by decarboxylation of Lysine | Shigella spp Providencia spp Pseudomonas spp H2S non-producing Salmonella spp |
| Red colonies with black centre | Xylose positive, lysine decarboxylase positive, capable of producing H2S,thus black centered colonies in alkaline pH | H2S producing Salmonella spp S. Typhi S. Typhimurium |
| Yellow opaque colonies | Ferment xylose but not lactose and sucrose, lysine negative, gives acid pH | E.coli, Klebsiella/Enterobacter Citrobacter, Serratia and Proteus spp |
| Yellow colonies | Lactose or sucrose fermentation, lysine negative, gives acid pH | Possible coliforms Sucrose positive Proteus spp |
Colony Characteristics of Different Microorganisms on Xylose Lysine Deoxycholate (XLD) Agar
| Organisms | Colony characteristics |
| Salmonella Typhimurium | red with black centres |
| Salmonella Abony NCTC | red with black centres |
| Escherichia coli ATCC 8739 | yellow |
| Escherichia coli NCTC 9002 | yellow |
| Escherichia coli ATCC 25922 (00013*) | yellow |
| Proteus vulgaris ATCC 13315 | grey with black centres |
| Salmonella Paratyphi A ATCC 9150 | red |
| Salmonella Paratyphi B ATCC 8759 | red with black centres |
| Salmonella Enteritidis ATCC 13076 (00030*) | red with black centres |
| Salmonella Typhi ATCC 6539 | red with black centres |
| Shigella dysenteriae ATCC 13313 | red |
| Shigella flexneri ATCC 12022 (00126*) | red |
| Shigella sonnei ATCC 25931 | red |
| Klebsiella aerogenes ATCC 13048 (00175*) | yellow |
| Enterobacter cloacae ATCC 13047 (00083*) | yellow |
| Staphylococcus aureus subsp. aureus ATCC 25923 (00034*) | inhibited |
| Enterococcus faecalis ATCC 29212 (00087*) | inhibited |
| Staphylococcus aureus subsp. aureus ATCC 6538 (00032*) | inhibited |
Quality Control for XLD Agar
Quality Control of XLD Agar involves assessing various parameters to ensure its performance and reliability. The following information provides insights into the quality control measures:
- Appearance: XLD Agar should have a light yellow to pink homogeneous free-flowing powder appearance.
- Gelling: The gelled medium should be firm, comparable to a 1.5% Agar gel.
- Color and Clarity of Prepared Medium: After preparation, the medium should form a red-colored clear to slightly opalescent gel in Petri plates.
- Reaction: A 5.67% w/v aqueous solution of XLD Agar should exhibit a pH of 7.4 ± 0.2 at 25°C.
- Cultural Response: The cultural response is assessed by incubating the plates at 35-37°C for a specified time period. Recovery rates and colony characteristics are observed and recorded for different organisms.
- Limitations: It is important to note the limitations of XLD Agar, including slight precipitation that may occur without affecting the medium’s performance. XLD Agar is a general-purpose medium that may not support the growth of fastidious organisms. False-positive reactions can occur due to the red to yellow coloration of some Proteus strains and the presence of red colonies in non-enteric bacteria like Pseudomonas and Providencia. Additionally, certain Salmonella species, such as S. Paratyphi A, S. Choleraesuis, S. Pullorum, and S. Gallinarum, may form red colonies without producing hydrogen sulfide, resembling Shigella species.
- Cultural Response: The cultural response for various organisms, including Salmonella Typhimurium, Escherichia coli, Shigella dysenteriae, and Enterococcus faecalis, is recorded based on growth observed, lot value, recovery percentage, and colony color.
These quality control measures ensure that XLD Agar performs as intended and provides accurate and reliable results for the isolation and differentiation of enteric pathogens.
| Positive control: | Expected Results (24 ± 3 hours at 37 °C) |
| Salmonella Typhimurium ATCC® 14028 * WDCM 00031 | Good growth; red colonies with black centre |
| Salmonella Enteritidis ATCC® 13076* WDCM 00030 | Good growth; red colonies with black centre |
| Negative control: | |
| Escherichia coli ATCC® 25922 * WDCM 00013 | Inhibited yellow colonies |
| Enterococcus faecalis ATCC® 29212* WDCM 00087 | No growth |
Uses of Xylose Lysine Deoxycholate (XLD) Agar
XLD Agar has several important uses in microbiology, particularly in the isolation and identification of Gram-negative enteric pathogens. Some key uses of XLD Agar include:
- Isolation of Enteric Pathogens: XLD Agar is commonly used for the isolation of Gram-negative enteric pathogens from fecal specimens and other clinical materials. It provides a selective environment that inhibits the growth of Gram-positive bacteria, allowing for the isolation of target pathogens.
- Differentiation of Shigella and Salmonella: XLD Agar is particularly suitable for the isolation and differentiation of Shigella and Salmonella species. The medium’s composition and indicator systems allow for the visual differentiation of these pathogens based on their ability to ferment xylose, decarboxylate lysine, and produce hydrogen sulfide.
- Microbiological Testing: XLD Agar is also used for microbiological testing of various samples, including foods, water, and dairy products. It enables the detection and enumeration of enteric pathogens in these samples, providing valuable information for quality control and ensuring food safety.
By providing a selective and differential environment, XLD Agar aids in the isolation, identification, and differentiation of specific Gram-negative enteric pathogens, particularly Shigella and Salmonella. Its applications in clinical and food microbiology make it a valuable tool for detecting and monitoring these pathogens in various samples.
Limitations of Xylose Lysine Deoxycholate (XLD) Agar
XLD Agar, while a useful medium, has certain limitations that should be considered. These limitations include:
- False Positive Reactions: Some strains of Proteus bacteria may produce red to yellow coloration on XLD Agar, accompanied by black centers. This can lead to false positive reactions, potentially causing misidentification of these bacteria as Salmonella or Shigella.
- Red Colonies by Non-Enterics: Non-enteric bacteria such as Pseudomonas and Providencia can exhibit red colonies on XLD Agar. This can create confusion during identification, as these organisms may resemble enteric pathogens.
- Red Colonies without Hydrogen Sulfide: Certain Salmonella species, including S. Paratyphi A, S. Choleraesuis, S. Pullorum, and S. Gallinarum, may form red colonies on XLD Agar without producing hydrogen sulfide. This resemblance to Shigella can complicate the differentiation between these pathogens.
- Prolonged Incubation: Incubation of XLD Agar plates for more than 48 hours may lead to false-positive results. Extended incubation can cause changes in the pH and color reactions of the medium, potentially affecting the interpretation of results.
Given these limitations, it is recommended that additional confirmatory tests be performed for complete identification. Biochemical, immunological, molecular, or mass spectrometry testing on colonies from pure culture can provide more accurate and reliable identification of the specific bacterial strains present. These supplemental tests help to overcome the limitations of XLD Agar and ensure accurate identification of enteric pathogens.
Storage and Shelf Life of Xylose Lysine Deoxycholate (XLD) Agar
Keep the prepared medium at 20-30°C and store between 10-30°C in tightly sealed containers. Make sure to use the product before the expiry date is printed on the label. To prevent product from clumping due to its hygroscopic nature, it should be stored dry after opening the package. Incorrect storage can lead to product lump formation. After use, store the product in a dry, ventilated place that is protected from extremes in temperature and ignition sources. Use the container before the expiry date printed on it.
Notes on XLD Agar
When working with XLD Agar, it is important to take note of the following considerations:
- Incubation Duration: Incubating XLD Agar for more than 48 hours can lead to false-positive results. It is recommended to make observations between 18 and 24 hours of incubation, as the color reactions may disappear after 24 hours. Extended incubation can cause alkaline reversion of normally acidic colonies, resulting in false positives.
- Avoid Prolonged Incubation: It is not advised to prolong the incubation period beyond the recommended time frame, as it can lead to inaccurate results and false positives due to changes in colony characteristics.
- Atypical Yellow Colonies: Some strains of Shigella (approximately 1%) may exhibit atypical fermentation of lactose, resulting in yellow colonies instead of the expected red color reaction on XLD Agar. This variation should be considered during interpretation.
- Red Colonies without H2S: Certain Salmonella species, including S. Paratyphi A, S. Choleraesuis, S. Pullorum, and S. Gallinarum, can form red colonies on XLD Agar without producing hydrogen sulfide (H2S). This characteristic can resemble the appearance of Shigella colonies. It is important to be aware of this similarity during identification.
By noting these considerations, one can ensure the accurate interpretation of results obtained from XLD Agar and minimize the risk of misidentification or false positives.
FAQ
What is the purpose of Xylose-Lysine-Deoxycholate?
Xylose Lysine Deoxycholate agar (XLD agar) is used to isolate Salmonella and Shigella species from clinical samples and food. Welton Taylor created the agar in 1965.
What is the principle of XLDA?
XLD agar preferentially increases the growth of Salmonella and Shigella by inhibiting other enteric pathogens, and differentiates Gram-negative enteric bacteria based on xylose fermentation, lysine decarboxylation, and hydrogen sulfide generation from sodium thiosulfate.
What is the composition of xylose lysine deoxycholate agar?
The components of XLD agar are yeast extract, sodium chloride, xylose, lactose, sucrose, l-lysine hydrochloride, sodium thiosulfate, iron (III) ammonium citrate, phenol red, sodium deoxycholate, agar, and distilled or deionized water.
Is Xylose-Lysine-Deoxycholate XLD Agar selective or differential?
XLD Agar, which stands for Xylose-Lysine-Deoxycholate, is a selective culture medium that can be used to isolate Salmonella and Shigella from food products or feces and other clinical samples. XLD Agar is a differential and selective medium. It includes yeast extract as a source of vitamins and nutrients.
What is XLD Agar used for?
XLD Agar is used for the isolation and differentiation of enteric gram-negative pathogens, particularly Salmonella and Shigella, in clinical, environmental, and food samples.
What are the selective agents in XLD Agar?
The selective agent in XLD Agar is sodium deoxycholate, a bile salt that inhibits the growth of gram-positive microorganisms.
How does XLD Agar differentiate between Salmonella and Shigella?
XLD Agar differentiates between Salmonella and Shigella based on their ability to ferment xylose, decarboxylate lysine, and produce hydrogen sulfide. Salmonella ferments xylose and decarboxylates lysine, resulting in red colonies with black centers due to H2S production. Shigella does not ferment xylose or decarboxylate lysine, leading to red colonies without black centers.
Can XLD Agar be used for other bacterial species?
While XLD Agar is primarily designed for Salmonella and Shigella, it can also support the growth of other enteric bacteria such as E. coli, Klebsiella, Citrobacter, and Proteus spp., which may exhibit characteristic colony colors and reactions.
How long should XLD Agar plates be incubated?
Observations should be made between 18 and 24 hours of incubation. Incubating the plates for longer than 48 hours may lead to false-positive results.
Can XLD Agar be used for environmental samples and food testing?
Yes, XLD Agar can be used for the microbiological testing of environmental samples and various food types to detect and enumerate enteric pathogens.
Are there any limitations to XLD Agar?
Some limitations of XLD Agar include potential false-positive reactions with Proteus strains, red colonies by non-enteric bacteria like Pseudomonas, and the possibility of red colonies without H2S production by certain Salmonella species.
What other tests should be performed for complete identification of isolated colonies on XLD Agar?
For complete identification, it is recommended to perform additional tests such as biochemical, immunological, molecular, or mass spectrometry testing on isolated colonies from pure culture.
How should XLD Agar be prepared?
To prepare XLD Agar, the agar powder is suspended in distilled water, heated until boiling, immediately transferred to a water bath at 50°C, and then poured into sterile Petri plates.
What is the purpose of the indicators in XLD Agar?
The indicators, including phenol red for carbohydrate fermentation and sodium thiosulfate/ferric ammonium citrate for H2S production, help differentiate bacterial species based on their metabolic activities and subsequent color changes.
References
- https://microbiologie-clinique.com/xld-agar-xylose-lysine-desoxycholate.html
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/419/519/14781dat.pdf
- https://www.himedialabs.com/media/TD/M031.pdf
- https://exodocientifica.com.br/_technical-data/M031.pdf