Viral Replication Definition
- Viral replication is the process of forming of biological viruses in the course of the infection process within the host cells.
- A virus has to go through the process of reproduction to generate new, infectious virions which are able to infect cells within the body and subsequent hosts.
- After entering the body, the virus makes physical contact and crosses its plasma membrane the target cell.
- Inside it, it creates and reproduces its genome and facilitates the production of its proteins through host ribosomes.
- The particles of a virus are derived from the biomolecules that have been synthesized and transform into infectious virus.
- Then, the virions are expelled from the cells in order to carry on the process of infection.
Role of nucleic acid – transfection
Higher organisms’ cells may be affected by viral nucleic acidthat produces normal viral ions. There are many important distinctions between the infection caused by nucleic acid (transfection) as well as viruses.
- The effectiveness of infection using nucleic acid is smaller, with a ratio of 10-6-10-8 in conventional media, which demonstrates how important the coat of viruses in the infectivity. The effectiveness of the virus can be improved by dispersal of viral DNA onto cells by calcium phosphate injection , or packaging into liposomes.
- Host range is larger with nucleic acids which are able to infect the cells that are resistant e.g. chicken cells, despite being not susceptible to poliovirus since they do not have receptors for virus, they are vulnerable to its RNA but only one cycle of multiplication by poliovirus occurs because the progenitors are also virions, and are unable to be transmitted to other cells due to the lack of appropriate receptors or other factors.
- Infectious nucleic acids can be extracted from viruses that have been heat activated where the capsid’s proteins is denatured. The nucleic acids can withstand more extreme temperatures than proteins. The capacity of nucleic acids infectivity to withstand any damage by the virus coat needs to be taken into consideration when preparing of vaccines against viruses.
- In the case of some RNA viruses DNA copies of the virus’s RNA is infective. e.g. polioviruses, which allows the production of viral genomes like vaccine strains in large quantities because it avoids the high rate of mutation in replication the RNA, and also its ability to replicate.
- The ability of nucleic acids to infect is not affected by viruses that are specific ABs This suggests that this type of virus may be a powerful infectious agent in the presence of immune system. But, the nucleases present in the body could restrict the role of nucleases. Of the animal viruses the adenoviruses and papovaviruses as well as herpesviruses as well as togaviruses and picorna generate infectious nucleic acid. Retroviruses can cause infectious DNA. may be isolated from cells infected or created by copying viral DNA in the laboratory.
Viral Replication Steps
All viruses must go through the seven steps to produce new virions. Certain stages can occur alongside other stages or even be performed out of sequence according to the virus. 7 stages for replication are in the following order:
- Attachment
- Penetration
- Uncoating
- Replication
- Assembly
- Maturation
- Release
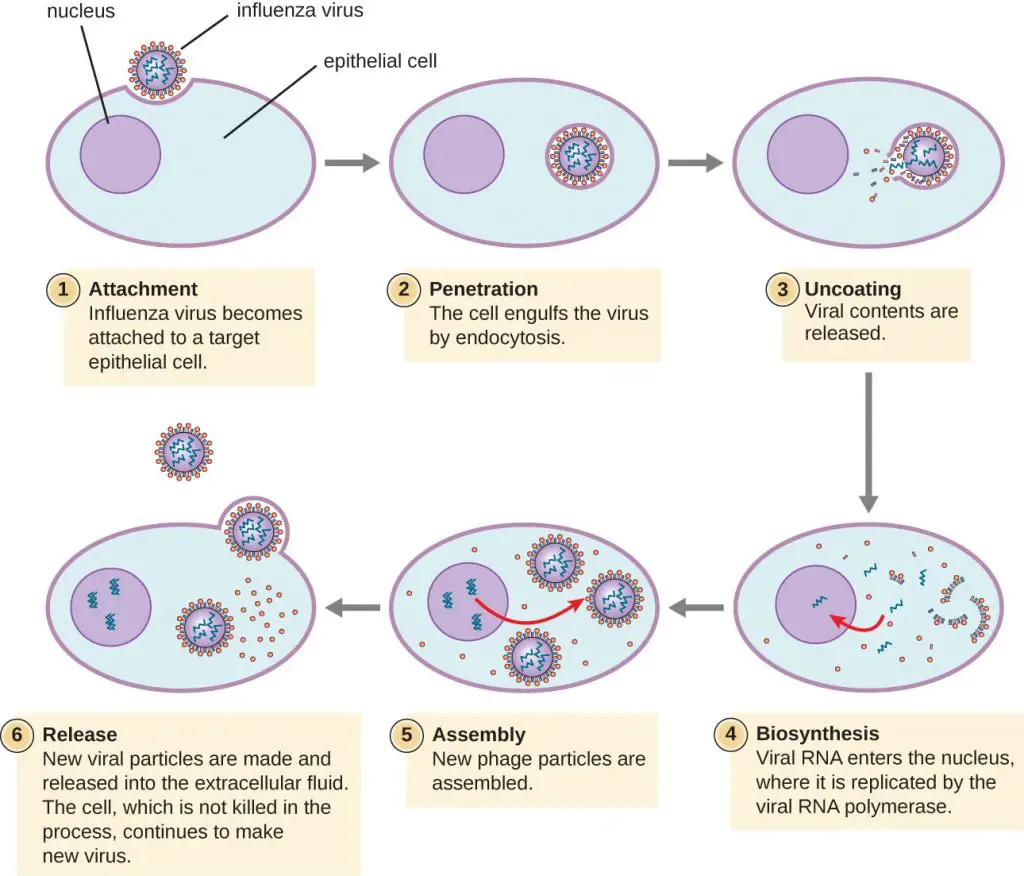
*Note: A good mnemonic to recall the stages of viral replication is the phrase “A PURple Apple Might Redden.” The letters in bold are the initial characters of each of the stages, in order.
1. Attachment of Viral Particles
- It is the initial step in the process of viral replication that the virus attaches onto the membranes of cells in the cell of the host.
- Plasma membranes of cells is comprised of a phospholipid bilayer which is characterized by a number of proteins sticking out through the membrane. The surface proteins perform various functions. These include transporting molecules and ions as well as facilitating the binding process of one cell to the other and acting as receptors to incoming proteins. Most plasma membrane proteins can be described as glycosylated which means they’ve been modified by carbohydrate and sugars.
- The receptor molecules that target the cell’s surface are typical proteins required for cell functions that viruses have developed to take advantage of, typically glycoproteins, or sugar and carbohydrate residues found on glycoproteins or on the plasma membrane.
- Certain viruses also require coreceptors to be able to infect cells. HIV initially attaches itself to a protein referred to by the name CD4 on the T lymphocytes’ surface (“T cells”) however it requires one of two proteins known as coreceptors to keep the infection process going.
Mechanism of Viral cell Attachment
- Attachment results from opposing electrostatic forces to the attachment protein of the virus and cells’ surface receptors. The attachment protein for the virus can be found in the exterior part of the virus because it is there that contact with the cell takes place.
- Attachment protein protrudes in the envelope of an enveloped virus unlike non-enveloped viruses, which have at least one capsid protein that communicate with cell-surface receptors.
- The attachment proteins of the virus can extend out from on the exterior of the virion, or may be contained inside “canyons” formed by capsid proteins.
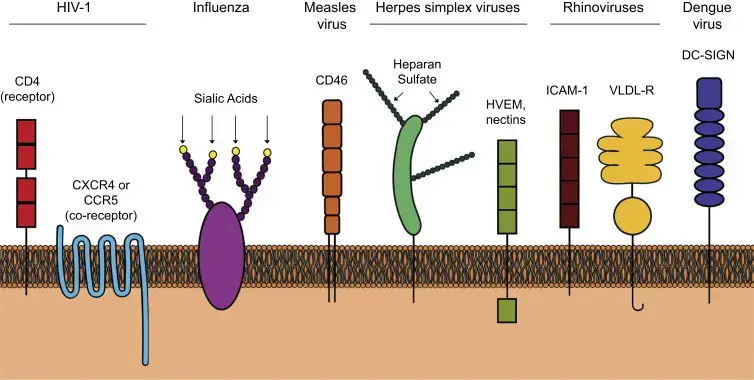
Cell surface receptors
Different viruses utilize specific receptors on the cell surface for attachment.
- In order to treat HIV-1, it is necessary CD4 to function as receptor, and the chemokine receptors CCR5 or CXCR4 as coreceptors.
- Influenza virus binds to sialic acid residues at the terminal located on cell surface glycoproteins.
- The measles virus laboratory strains are able to bind CD46 (although CD150 also acts as a receptor for measles virus).
- Herpes simplex virus-1 first is bound to heparan sulfur on GAGs in order to connect to entry receptors, for example, HVEM and nectins.
- Ninety percent of rhinoviruses employ ICAM-1 as a receptor while 10% of rhinoviruses use that VLDL receptor.
- Dengue virus is transmitted through DC-SIGN.
Cell Surface Receptors for Attachment of Human Viruses
| Virus | Cell surface receptor(s) |
| Rhinoviruses | Intercellular adhesion molecule 1 (ICAM-1) (90%), low-density lipoprotein receptor (10%) |
| Poliovirus | Poliovirus receptor (PVR) CD155 |
| Human immunodeficiency virus | CD4 (receptor); CCR5 or CXCR4 (coreceptors) |
| Influenza A virus | Sialic acid |
| Measles virus | CD46, CD150 |
| Herpes simplex virus-1 | Heparan sulfate, HVEM, Nectin-1 |
| Dengue virus | DC-SIGN |
| Hepatitis B virus | Sodium taurocholate–cotransporting polypeptide |
| Human papillomavirus | Heparan sulfate, integrins |
2. Penetration
- After attachment, the successful virus quickly enter the cell , allowing it to escape any extracellular stress that could damage the virion, for example the movement of mucus.
- Penetration is the process of crossing your plasma membrane caused by viruses. Contrary to attachment to the virus penetrating requires energy but this is provided through the cells of the host, but not by the virus.
Mechanism of Viral Penetration
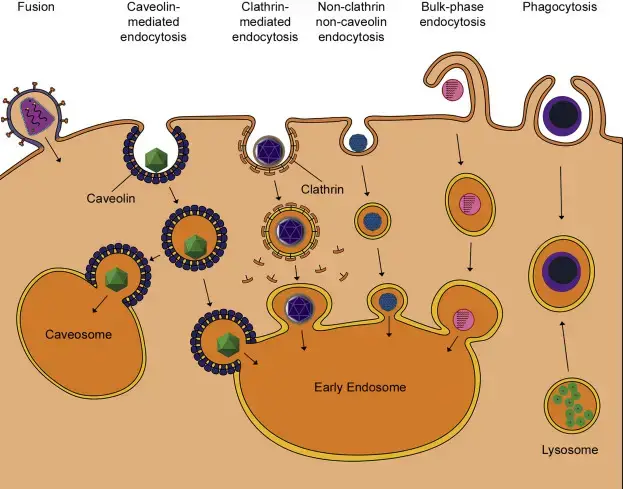
Different viruses utilize diverse cell-based mechanisms to enter the cell by binding to specific receptors on the cell surface.
- Receptor-mediated endocytosis: Receptor-mediated Endocyto is typically utilized by viruses to enter the membrane of plasma. When the pH of an endosome decreases, virus proteins alter their configuration that allows them to be released out of the endosome. Based on what virus is at play, it could occur in early endosomes, the late endosomes or Lysosomes. Both enveloped and non-enveloped viruses benefit from receptor-mediated endocytosis for access into the cytoplasm the cell. Both enveloped and non-enveloped viruses make use of receptor-mediated endocytosis within pits that are coated with caveolin or clathrin.
- Clathrin-mediated endocytosis: The majority of viruses utilize clathrin-mediated endocytosis to enter cells such as dengue virus, hepatitis C virus and Reoviruses.
- Caveolae-mediated endocytosis: A handful of known viruses that affect human beings, like SV40 and the papillomaviruses (that cause warts and cervical cancer) make use of caveolae-mediated endocytosis and this was found out making use of a medication that blocked the development of caveolae. Eliminating clathrin-mediated endocysis did not stop the entrance of the virus into cell. Some viruses are also subject to endocytosis mediated by receptors that is indistinguishable from both caveolin and clathrin.
- Bulk-phase endocytosis: During bulk-phase endocytosis the cell creates an enveloping vesicle to engulf whatever molecules are in the extracellular fluid, which includes viruses.
- Phagocytosis: Phagocytosis is a form of endocytosis that is mediated by receptors and is used by cells that are specialized to completely engulf cells. Recently, two massive DNA-based viruses, including HSV-1 as well as Mimivirus were found to enter cells via phagocytosis pathways.
- Fusion: Fusion is a method of penetration only used by enveloped virus is the fusion. The viral envelope fusion is possible at the cell membrane, or in endocytosed vesicles like the endosome. It is controlled by the same virus protein used by the virus to attach and attachment, or via a distinct virus protein, based on the virus.
| Type of penetration (entry) | Virus examples |
| Clathrin-mediated endocytosis | Dengue virus, hepatitis C virus, reovirus, adenovirus, parvovirus B19, West Nile virus |
| Caveolin-mediated endocytosis | Human papillomavirus, SV40, hepatitis B virus |
| Fusion | HIV, influenza, respiratory syncytial virus, herpes simplex viruses, dengue virus, Ebola virus |
3. Uncoating
- Uncoating is the term used to describe the breaking down or elimination of the capsid causing an exodus of virus’s genome to the cell the place where genome replication and transcription occur.
Mechanism of Uncoating
Uncoating is a process that can be distinguished from or closely linked to penetration. Viruses can also achieve the process of uncoating through a variety of different methods.
- Rhinoviruses: rhinoviruses get transported into cells via endocytosis mediated by receptors that are clathrin-co. Within the acidic endosomes, the virus grows in size by four percent. One capid protein, the VP1 (viral protein 1) is able to form pores within the endosome, which allow for the release of rhinovirus genome.
- Influenza virus: The influenza virus contains a virus-specific protein known as hemagglutinin (HA) in the envelope of the virus. It binds with sialic acid residues in the outer surface of respiratory epithelial cell and enters the cell via endocytosis that is mediated by receptors. The pH drop of the endosome results in changes in the shape in the virus’s HA protein, which reveals an fusion peptide that draws the two membranes closer and connects the viral envelope with the membrane of the endosomal. In this case, the HA protein aids in attachment and the removal that the virus. The viral DNA genome segments are carried into the nucleus before entering through the nuclear pores.
- Other viral capsids(Poliovirus): The poliovirus capsids has been believed to be unable to enter cells in any way: the presence of the capsid poliovirus with the cell-surface receptor results in an alteration in the virion, which creates an opening inside the cell membrane through which the viral RNA can be released into the cells’ cytoplasm. Many viruses keep a capid that is intact inside the cytosol which serves as an “home base” for replication similar to reoviruses.
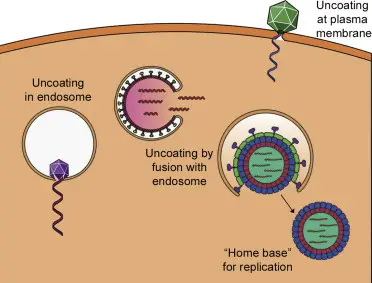
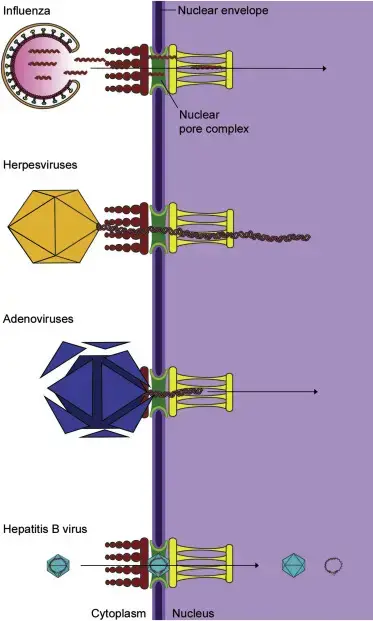
Transport of viral genomes into the nucleus.
A variety of viruses need to transport their genomes to the nucleus in order for replication or transcription to take place. Influenza genome segments are transported through the nuclear pore to the nucleus. Capsids of herpesvirus are transported via microtubules into the nuclear pore, from where the uncoating takes place. The capsids of adenovirus break up in the nuclear pore, and virus DNA gets then transported to the nucleus. Others viruses like hepatitis B virus are so small that the whole capsid may be able to traverse the nuclear pores.
4. Replication
Viral viruses, whether DNA or RNA, shut off the process of synthesis of protein in cells and break down cellular polyribosomes leading to a shift towards the synthesis of viral proteins. The mechanism for shut-off of protein synthesis varies across the virus family.
Poliovirus, utilizing an enzyme that is viral, triggers the cleavage of a 200Kd cap-binding protein that is essential for the initiation of the process of translating capped cellular messengers. In contrast to viruses with virulence mild virus e.g. polyomaviruses can stimulate the production of host DNA and mRNA and proteins. This phenomenon is of particular interest for viral carcinogenesis.
DNA Viruses
For DNA viruses in animals transcription and translation do not get connected. Except for poxviruses, transcription takes place within the nucleus and the translation takes place within the cells. Generally, the first transcripts produced from RNA Polymerase II are greater than the mRNAs on ribosomes. In some instances there is a possibility that as much as 70% of transcriptional RNA is not translated in the nucleus.
The messengers of the virus but, as with animals’ cells can be unicentric. Transcription has an orderly temporal structure, and for the majority of DNA viruses having only a tiny fraction of their genome being transcribed to the early messages. The synthesis of proteins in the early stages is the primary stage in viral DNA replication.
Following DNA synthesis, the remaining genome is transscribed in late-stage messages. The complex viruses possess immediate early genes that are activated in the presence of proteins that inhibit protein production as well as delayed early genes which require protein synthesis expression.
Regulation is accomplished through proteins found in virus, or as specified by cellular or viral genes, which interact with regulatory sequences located at the 5′-end of gene. These sequences could react in trans to the products generated by other genes and also act in cis with the genes associated with it. Different types of genes can be transscribed from different DNA strands and thus in opposing orientations e.g. Polyomaviruses. The transcripts can undergo post-transcriptional processing to ensure that any non-essential intervening sequences are eliminated.
DNA replication
The method for replication can be described as semiconservative however how the intermediates that replicate depend on the mode that replication is carried out. Several methods of replication can be identified.
- Adenoviruses: Adenoviruses have Asymmetric replication that starts at the 3′-end in one of the chains, using the protein primer. The growing one strand will displace the previous one with the same polarity and creates the complete double structure. The displaced strand then replicates in a similar way by forming a panhandle by combining with the reversed terminal repetitions.
- Herpesviruses: It has linear genomes that have terminal repeats. On reaching the nucleus these terminals undergo a limited exonucleotic digestion, and they then create circular. Replication is thought to occur through the rolling circle mechanism in which concatemers are created. During maturation units-length molecules are separated from concatemers.
- Papovaviruses: The DNA sequences of papovaviruses is circular, and replicating process is bidirectional as well as symmetrical through intercalations that are cyclic.
- Parvoviruses: The replication of single stranded parvoviruses started when +ve and -ve Stranded DNA from various parvoviruses join together to form a double-stranded DNA molecule. It is from this that replication and transcription take place.
- Poxviruses: The distinctive characteristic of the poxvirus DNA is that the two strands of DNA connect. The replicative intermediates, which are found in the cytoplasm, are concatemers, which contain genomes that are connected head-to-head or tail in tail.
- Hepadnaviruses: Hepatitis B virus uses reverse transcription to replicate. The genome is composed of a partially double-stranded circular DNA, with an entire negative strand as well as an uncomplete positive. Upon entering the cell the positive strand becomes completed and transscribed. RNA transcripts are then reverse-transcribed into DNA by a virus enzyme, in a series of steps, closely following the pattern of retroviruses which includes a leap of the new positive line to a directly repeated (DR) in one direction to another.
RNA Viruses
Replication of the RNA viral genome is determined due to the lack of translation units in the messenger, which is a characteristic of all cell messengers in animals. To overcome this issue, three main strategies have been devised.
- This mRNA from the virus functions directly as a messenger which is translated monocistronically followed by cleavage into various proteins.
- The virion’s DNA can be transformed to yield a variety of monocistronic monocistronic MRNAs by starting transcription in various locations.
- The genome is an assortment of different fragments of RNA that are transscribed to monocistronic MRNA.
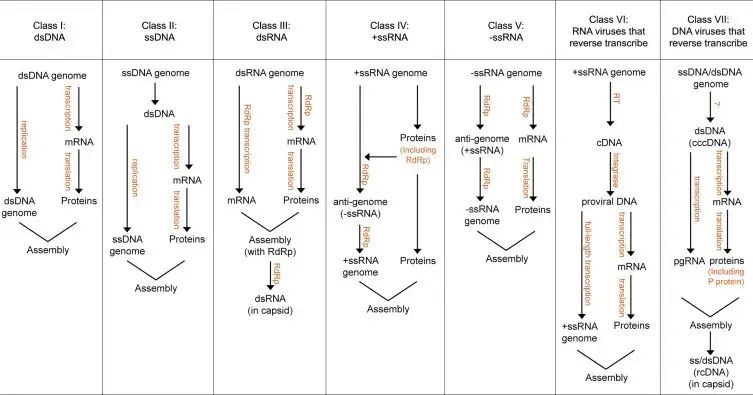
The RNA viruses can be classified into seven classes depending on what the RNA in the virus is as well as its relationship with its messenger.
1. Class I (e.g. picornaviruses, flaviviruses.)
A genome with +ve polarity, acts as a messenger, indicating information about the synthesis of structural and non-structural proteins.
This RNA molecule can also trigger replication that requires protein expression first. This format gives little control over the replicating e.g. Poliovirus is not able to independently control controlling the number of structural proteins produced.
2. Class II (e.g. coronaviruses, togaviruses.)
Many +stranded RNA viruses contain subgenomic RNA within the cycle. This gives them some degree of control. Subgenomic mRNA can’t be detected from the RNA Polymerase. It could be utilized for the sole purpose of production of structural proteins. Another method to overcome the issue is to create the nesting set of RNAs. The nested set of RNAs is the most effective form that controls. They can decide the part of their genome they express.
Togaviruses are responsible for the translation of it is the case that the genome-wide RNA of 49S first gets transformed into polyproteins that are transformed into non-structural protein. A subgenomic mRNAthat is transscribed from the entire length -ve RNA is transformed into a smaller polyprotein which is transformed into structural proteins of the virus. Coronaviruses produce the nested set of mRNAs are generated by following the same process that is, the -ve transcript initially generated from the genome. This transcript is then transformed into monocistronic monocistronic monocistronic MRNAs dimensions. Each one begins with the same 5-foot-long leader sequence that is connected to the transcripts near the beginning of the different genes and continues until the 3′-end in the genome. These mRNAs cannot be made by splicing the genomic size transcript since the virus can reproduce in enucleated cells.
3. Class III (e.g. paramyxoviruses, rhabdoviruses.)
It is of a polarity of -ve with respect to its Messenger. A virion RNA-dependent RNA transcriptionase initially transcribes the genomes into monocistronic signals that start from one promoter. The transcriptase ceases and resumes at every junction between the different genes.
4. Class IV (e.g. orthomyxoviruses, most bunyaviruses.)
The -ve genome can be found in various distinct, non-overlapping segments of ssRNA. Virion transcriptase creates one messenger for each piece. Orthomyxoviruses are the most common. genomic segments have one gene, but two fragments have two genes that are overlapping that are expressed through a full-length messenger while the other one is expressed through an a smaller messenger derived from the first through the process of splicing. The replication process of orthomyxoviruses is distinct from other the RNA viruses as it occurs in the nuclear nucleus. The nuclear function that it needs to function is through the 5′ caps of cellular messengers that can be found in “pinches” after endonucleotic cleavage of hosts’ messengers. This 5′-cap is utilized as a primer in the creation of viral messengers.
5. Class V (e.g. arenaviruses, phleboviruses.)
Arenaviruses possess an ambisense genome. This means that half of the genome is of polarity -ve and is translated into a message by the virion transcriptase. However, the other halfthat contains +ve,, gets being transcribed twice. First, an entire transcript of the genome is created, and then the mRNA gets transcribed into the transcription. This strategy is evident in the (small) (small) portion in the genomes of Phleboviruses. Ambisense genomes are not common for RNA viruses, but not for dsDNA viruses.
6. Class VI (e.g. reoviruses. Reoviruses.)
Have distinct, non-overlapping segments of DsRNA. Each segment is transscribed into an mRNA that is independent through the virion transcriptase. The majority of messengers are monocistronic. But one is bicistronic , and it expresses another protein, which begins at an internal AUG , in an entirely different reading frame. Every segment of reovirus DNA is replicated separately. A new mRNA strand is created by virion transcriptase. This strand is then used as a template for the replicase that will create the negative one. The two strands stay attached to a dsRNA-containing molecules that end up in virion. The replication process is asymmetrical and conservative due to (1) the virion -ve strand serves as the RNA servers is used as the initial template, and (2) the parent RNA is not incorporated into the progeny.
7. Class VII (e.g. retroviruses.)
Retroviruses differ from other retroviruses in that their genomes have been transscribed into DNA, not an RNA. They contain two identical ssRNAs with +ve polarity. There is the poly A tail at the 3′-end and an end cap at the 5′-end. Each is transscribed into DNA using reverse transcriptase and then integrated into the DNA of the cell, forming a provirus. Transcription of the provirus by the cell transcriptase results in the formation of viral molecules which end up in the form of virions.
Because RNA viruses belonging to classes III through VII require virion transcriptase to synthesize a messenger, their purified viral RNAs aren’t virus-like. Only those of class I, II and III are infected. With RNA viruses there is no distinction between late and early messengers.
Replication of Single-Stranded RNA Viruses (Classes I to V)
In all instances replication is the process of creating the template strand which is similar to the viral one of the same length and serving as the basis for progeny viral Strands. These steps are executed by a variety of enzymes, both of the cellular and viral nature, along with the nucleocapsids that infect the virions.
In many cases replication and transcription can interfere with one another: when stranded viruses are -ve, transcription and template are made of viral strands. With (+ve) viruses, the virus strand could be used as a mediator or a replication templates. Initially in the virus there is no interference because the function of messenger is to supply proteins required to replicate.
In the future, the proteins’ supply will regulates the rate at which replication occurs. e.g. in the case of the poliovirus virus, replication begins when the pVg proteins are covalently bound to the 5′ end of the RNA, presumably creating an RNA replication complex. Messengers and their progeny frequently differed in their structural features. e.g. The influenza virus’s messengers possess cap-capped leader sequences, which are that are derived from cell messengers.
Additionally that they are deficient in 17-22 nucleotides at the 3′-end. Moreover, replication requires continuous protein synthesis in order to produce the necessary proteins, while transcription doesn’t.
For RNA replication the newly created template strand is able to remain attached to the viral strand upon the basis of which it is constructed and forms a double-stranded strand that runs the length of the viral genome, also known by the term replicative (RF). Synthesis of new strands is accomplished via asymmetric synthesis that is conservative, like the adenoviruses.
An RF that is associated with an infancy viral strand is also known as the RI (replicative intermediate) The RF molecules are quite abundant in replication since following the completion of an entirely new strand, replicase are believed to remain connected for a period of time with the template prior to resuming the process of synthesis. RFs accumulate in the course of replication when there are no more RIs to be formed.
Except for orthomyxoviruses. The RNAs of the virus reproduce in the cells’ cytoplasm. The replicase that are present in cells infected with the virus create new strands of viral RNA of both opposite polarities. Transcription occurs in the same location that replication occurs. It is unclear if transcription and replication are accomplished by distinct enzymes or through one enzyme.
5. Assembly
- Viruses originate by synthesizing new components and, in order for them to escape from cells, these components must be gathered at a specific location within the cell, and then are assembled into an embryonic virus.
- Similar to the way penetration and uncoating are hard to separate within the cycle of certain viruses, assembly is often able to occur during the process of maturation as well as release.
- The place of assembly of virion will be determined by the specific virus. It may occur in the cell’s nucleus or at the plasma membrane or on various intracellular membranes like that of Golgi complex.
- The majority of DNA viruses that are not enveloped assemble their nucleocapsids in the nucleus since it is the place where genome replication. Viral proteins are imported via the nuclear pores before reaching the assembly site. After assembly, many DNA virus are massive to pass through the nuclear pores, but. As of now, certain viruses can pass through the double-membraned nuclear membrane and some trigger cell death or apoptosis, which allows them to get out of the nucleus.
- On the other hand viruses with envelopes that originate from plasma membranes usually are grouped there.
- Nucleic acids in the genomes of viruses such as a helical virus protected by repeated capsid proteins. This is why capsid proteins may wrap the genome once the genome is copied (or in reverse dependent on how the virus is conceived: genome could wrap around capsid proteins).
- Contrary to this, certain Icosahedral viruses are close to completing the assembly of their capsids prior to the nucleic acids genome is added.
- The spontaneous assembly of the capsid called “self-assembly,” occurs with the capsid proteins from simple icosahedral virus species, like picornaviruses or parvoviruses.
- A virus’s assembly that have more complex architecture is controlled by a range of chaperone proteins for viruses called scaffolding proteins.
- Adenoviruses and herpesviruses are two an example of huge icosahedral virus which assemble with scaffolding protein aid.
6. Maturation
- Once the nucleic acid genome as well as other proteins are contained within the capsid, constructed from one or more translational viral proteins. The next steps of replication take place maturation and release.
- At this point, the virion been developing as a cell. If the cell was broken open after this time, virion would be in no position to start infecting new cells.
- Maturation refers specifically to the final changes in an immature virion, which result in the formation of an infectious virus particle. Capsid structural changes are typically involved, and they are mediated by host enzymes or virus encoded enzymes.
Mechanism of Maturation
Influenza Virus
A great example is one of them being the influenza H protein. It’s involved in attaching with the cells sialic acids, as mentioned above as well as the protein has the ability to bind to sialic acid after having been glycosylated (via posttranslational modifications). The protein must be broken down into two parts that are HA1 and the other for it to become infectious. This is because even though the HA1 portion is able to bind cells’ surface receptors, the other part is the one that binds the envelope of the virus to the membrane of the endosomal cell to release viruses into cells’ cytoplasm. The cleavage of the HA protein into HA1 and HA2 occurs through cell proteases (enzymes which cleave proteins).
HIV
Contrary to this it is the HIV core particle is made up from the gag genes encoded proteins. The gene is transformed into a polyprotein which is broken down via the HIV protease in order to create the matrix, capsid and nucleocapsid proteins that comprise the virion. In this scenario maturation takes place when the virion is removed from the cell surface, and is necessary for the formation of an infective virion. In Chapter 8 “Vaccines, Antivirals, and the Beneficial Uses of Viruses,” many anti-HIV medicines function by preventing the actions of the HIV protease, which prevents the cleavage of the protein and subsequent creation infective virion.
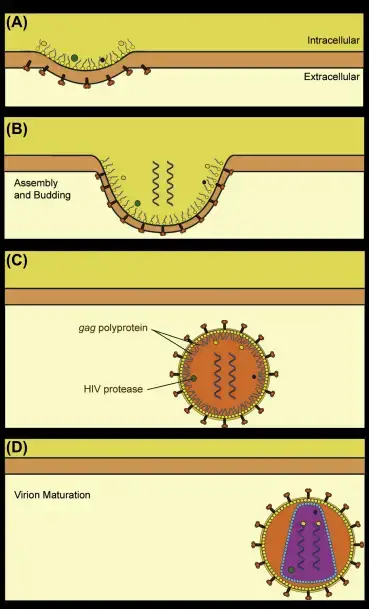
Assembly, maturation, and release of HIV Violons occurs as a result of the following steps:
- (A) HIV proteins congregate on the plasma membranes of cells and cause a bud to develop in the membrane.
- The DNA of the diploid genome is packaged inside the capsid that assembles (B).
- The virus has been dispersed by the membrane of the cells however, the Gag polyprotein has not been broken down to separate the matrix and capsid proteins from the virion (C).
- The HIV protease cuts the polyprotein and allows the proteins to build the virion’s structure (D).
7. Release
- The final stage in the cycle of replication is the release from the virion to the surrounding environment in order to continue to infect by releasing new cells.
Mechanisms Involved in Release
The release can happen in many different ways, based on the virus.
Budding
- Viruses that get their envelopes from the plasma membrane usually are able to form an envelope on the inner layer of plasma membrane, encapsulating their envelope proteins in the plasma membrane.
- When the capsid proteins of the virus are in contact with the membrane-associated viral proteins, they create a plasma membrane that begin to curving over the capsid.
- The process continues up to the point that plasma has completely enclosed around the virus and is when the cell ceases to exist. This is called budding.
- The virus can develop from any membranes in the cell such as the rERsystem, Golgi complex and even nuclear envelope.
Exocytosis
- In this instance the virion that is already enveloped doesn’t require budging via to the plasma membrane. It is usually exocytosed to let go of the cell.
- The non-enveloped virus can also exit the cell through exocytosis.
Lysis
- Lytic viruses cause disruption to the plasma membrane, and trigger the lysis or burst in the cells. The virus then releases its nascent form to infect the new cells.
- Human viruses that are not enveloped are released via cell lysis.
References
- Louten J. Virus Replication. Essential Human Virology. 2016:49–70. doi: 10.1016/B978-0-12-800947-5.00004-1. Epub 2016 May 6. PMCID: PMC7149683.
- https://virology-online.com/general/Replication.htm
- https://en.wikipedia.org/wiki/Viral_replication
- https://www.immunology.org/public-information/bitesized-immunology/pathogens-and-disease/virus-replication
- https://courses.lumenlearning.com/boundless-microbiology/chapter/viral-replication/
- https://www.news-medical.net/health/How-does-Viral-Replication-Work.aspx
- https://www.sciencedirect.com/topics/medicine-and-dentistry/virus-replication
- https://www.frontiersin.org/articles/10.3389/fmicb.2018.01546/full