What is Trichinella spiralis?
- Trichinella spiralis is a viviparous nematode parasite recognized for its role in causing the disease known as trichinosis. This parasite predominantly inhabits a range of hosts, including rodents, pigs, bears, and hyenas, as well as humans. Commonly referred to as the “pork worm,” it is primarily associated with the consumption of undercooked pork products, though it can also be found in the meat of other infected animals. It is crucial to distinguish Trichinella spiralis from the unrelated pork tapeworm, as they belong to different taxonomic groups.
- The life cycle of Trichinella spiralis is particularly noteworthy due to its complexity and adaptability. Once ingested by a definitive host, such as a pig, the small adult worms mature in the small intestine. Here, the females produce live larvae, which are released into the intestinal environment. These larvae then penetrate the intestinal wall, entering the host’s bloodstream and lymphatic system. They travel throughout the body, ultimately reaching striated muscle tissues, where they encyst. This encystment process involves the larvae being encapsulated in a fibrous tissue capsule, which provides a protective environment for the parasite while it remains dormant.
- Humans typically contract trichinosis by consuming undercooked or raw meat containing these encysted larvae, particularly pork, but also horsemeat or wild carnivorous animals such as foxes and bears. Upon ingestion, the larvae are released from their cysts and begin their life cycle anew, maturing into adult worms in the human intestine.
- The clinical implications of Trichinella spiralis infections are significant. Trichinosis can lead to a variety of symptoms, including gastrointestinal disturbances, muscle pain, and systemic reactions as the larvae migrate and invade muscle tissue. The severity of the infection often depends on the number of larvae ingested, with heavier infections resulting in more pronounced symptoms and potential complications.
History and Distribution of Trichinella spiralis
Trichinella spiralis is a tissue nematode known as the causative agent of trichinosis, a significant zoonotic disease impacting human health. Its historical context and distribution reveal critical insights into its epidemiology and the public health challenges it poses.
- The term Trichinella is derived from the Greek word trichos, meaning “hair,” reflecting the minute size of the adult worm, while the suffix ella indicates a diminutive form. The name spiralis highlights the characteristic coiled appearance of the larvae found in muscle tissue.
- The earliest documented observation of Trichinella spiralis occurred in 1821. James Paget, a first-year medical student at St. Bartholomew’s Hospital in London, identified the nematode in the muscle tissue of a deceased patient during an autopsy. This discovery marked the initial understanding of the parasite’s role in human pathology.
- In 1835, the naturalist Richard Owen further contributed to the knowledge of Trichinella by describing its encysted larval form within muscle tissues and formally naming it Trichina spiralis. His work laid the foundation for the scientific understanding of this parasite’s life cycle and pathogenicity.
- The life cycle of Trichinella spiralis was elucidated by Rudolf Virchow in 1859, providing essential information on how the parasite infects its hosts. The life cycle begins when larvae are ingested through contaminated meat. Once in the host’s intestine, they mature into adult worms, reproduce, and their larvae subsequently migrate to striated muscle tissue.
- The primary source of human infection is linked to the consumption of undercooked or raw pork, which may contain encysted larvae. This connection underscores the importance of proper food handling and cooking practices to prevent transmission of the parasite.
- Trichinosis has emerged as a significant public health concern, particularly in Europe and North America, where cases have been reported in various populations. However, it is notably less prevalent in tropical regions and many parts of Asia. The patterns of transmission can vary based on cultural dietary practices and food safety regulations.
- In India, the first recorded case of human trichinosis was only documented in 1996 from the Punjab region, indicating that the presence of the parasite in local wildlife or livestock had not previously been recognized as a public health issue. This highlights the dynamic nature of zoonotic diseases and the need for ongoing surveillance.
- Overall, the distribution of Trichinella spiralis is closely associated with swine production and dietary habits. Regions with high pork consumption, particularly where meat is often prepared in insufficiently cooked forms, experience higher incidences of trichinosis.
- Understanding the historical context and distribution of Trichinella spiralis is crucial for developing effective public health strategies aimed at reducing infection rates. Educational campaigns focusing on the importance of cooking meat thoroughly, as well as regulatory measures for food safety, can significantly mitigate the risk of trichinosis outbreaks.
Habitat of Trichinella spiralis
The habitat of Trichinella spiralis is intricately tied to its life cycle and the hosts it infects. This nematode parasite relies on specific environments within its hosts for development and reproduction, demonstrating remarkable adaptation to its biological niche.
- Primary Habitat: Adult Trichinella spiralis worms inhabit the mucosal layer of the small intestine, primarily residing in the duodenum or jejunum of various hosts, including pigs, bears, rats, and humans. This specific habitat provides a suitable environment for the worms to mature and reproduce, benefiting from the nutrient-rich surroundings of the intestinal tract.
- Infection Mechanism: Upon ingestion of contaminated meat containing encysted larvae, the larvae are released in the intestine and develop into adult worms. They embed themselves within the intestinal mucosa, where they can absorb nutrients and grow. The intimate association with the host’s tissues is critical for their survival, as they do not have free-living stages in the environment.
- Larval Encystment: After mating, female worms produce live larvae that penetrate the intestinal wall and enter the bloodstream. This transition marks a significant phase in their life cycle, as the larvae travel to the striated muscle tissues of their hosts. Here, they encyst, forming a protective capsule around themselves. This encystment allows the larvae to survive in a dormant state, often for years, until consumed by a new host.
- Muscle Tissue Habitat: The encysted larvae can be found in various striated muscles, including those of the diaphragm, tongue, and other skeletal muscles. This location is essential for their continued propagation. When the encysted larvae are ingested by another host, they can exit the cysts and reinfect the intestinal tract, thereby continuing the life cycle of Trichinella spiralis.
- Host Distribution: The habitats of Trichinella spiralis are predominantly found in mammals that consume contaminated food sources. Pigs are significant hosts in agricultural settings, where undercooked pork products pose a high risk for human infections. Additionally, wild carnivores such as bears and rats can serve as reservoirs, contributing to the persistence of the parasite in various ecosystems.
- Absence of Free-Living Stages: Unlike many other nematodes, Trichinella spiralis does not have free-living larval or adult stages in the external environment. This lack of free-living forms necessitates a direct lifecycle reliant on specific host organisms, making the habitat entirely dependent on the presence of suitable hosts.
- Environmental Factors: The transmission of Trichinella spiralis is heavily influenced by environmental conditions that affect the survival and encystment of larvae. For instance, factors such as temperature, humidity, and the presence of suitable host animals play crucial roles in maintaining the lifecycle of this parasite.
Morphology of Trichinella spiralis
The morphology of Trichinella spiralis is essential for understanding its life cycle and pathogenicity. This nematode parasite exhibits distinct structural features that facilitate its survival within host organisms, enabling effective infection and reproduction.
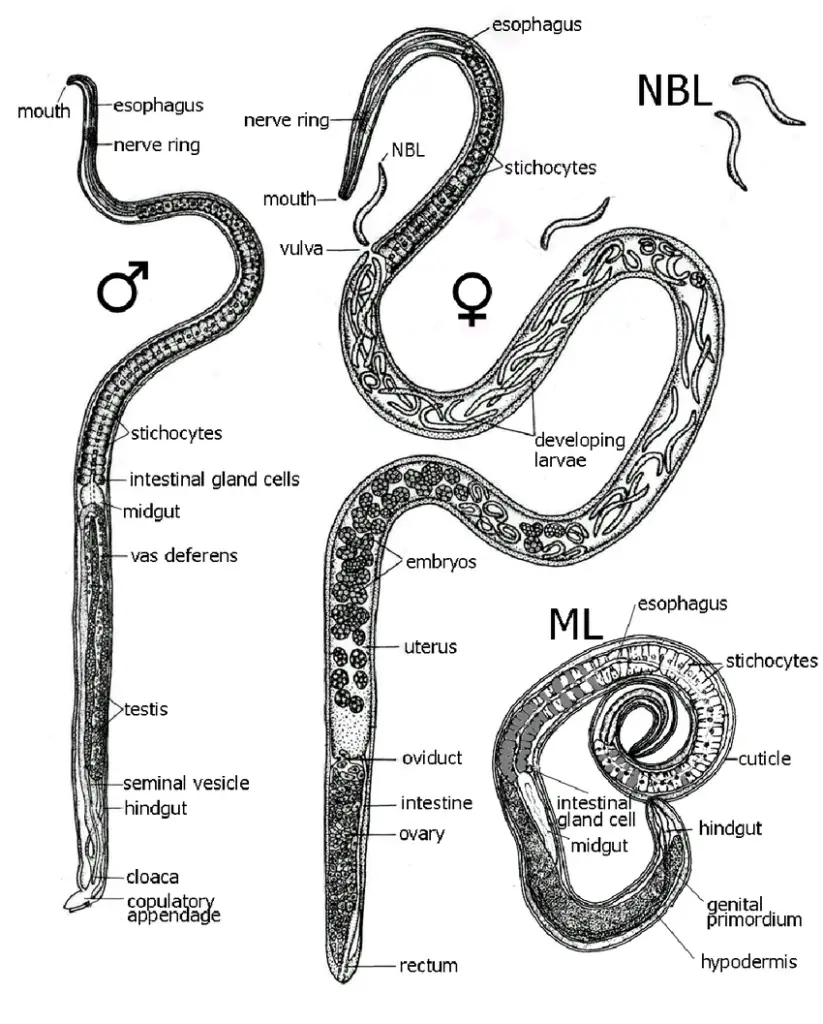
- Adult Worm Characteristics:
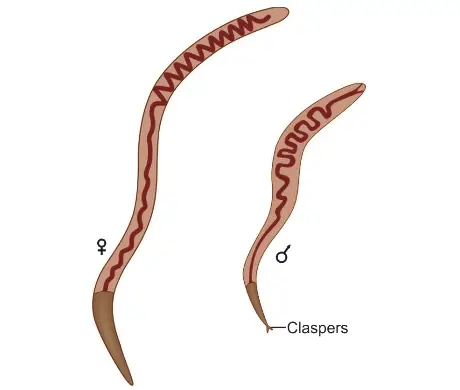
- The adult T. spiralis is a small, white nematode, one of the tiniest roundworms that infect humans. It is barely visible to the naked eye, with males measuring approximately 1.5 mm in length and 0.04 mm in diameter, while females are larger, at around 3 mm in length and 0.06 mm in diameter. This size difference, where females are nearly twice the length of males, is notable among nematodes.
- The anterior half of the adult worm is slender and pointed, which is an adaptation that aids in burrowing into the mucosal epithelium of the host’s intestine. This morphology is crucial for the worm’s ability to anchor itself securely within the intestinal lining, facilitating nutrient absorption and protection from host defenses.
- The posterior end of the male possesses a pair of pear-shaped clasping papillae, referred to as claspers, located near the cloacal orifice. These structures are used during mating to grasp the female worm securely, ensuring successful fertilization.
- Reproductive Strategy:
- Female T. spiralis are viviparous, meaning they give birth to live larvae rather than laying eggs. This reproductive strategy allows for a more immediate establishment of the parasite within the host’s tissues.
- The lifespan of adult worms is notably short. Males typically die shortly after mating, while females have a lifespan ranging from 4 weeks to 4 months (approximately 16 weeks), which corresponds to the time required for them to discharge larvae into the host’s bloodstream.
- Larval Characteristics:
- Upon the release of larvae from the female, they migrate through the bloodstream to striated muscle fibers, where they undergo encystment. At the time of encystment, these larvae measure about 1 mm in length and 36 μm in diameter. Their coiled shape gives rise to the name spiralis, reflecting their distinctive morphology.
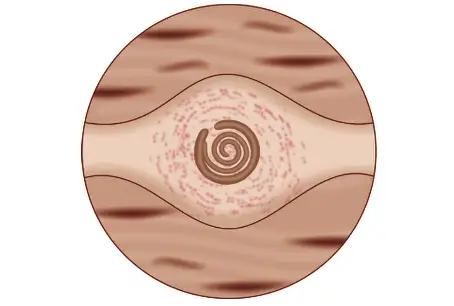
- Trichinella Cysts:
- The encysted larvae form ovoid cysts measuring approximately 400 μm by 250 μm. These cysts result from the host’s tissue reaction to encapsulate the larvae, creating a protective environment.
- Cysts typically develop in muscle tissues that are poor in glycogen and exist in hypoxic environments. As a result, muscles that are constantly active, such as the diaphragm, biceps, jaw muscles, extraocular muscles, neck, and lower back, are primarily affected. These locations are advantageous for the parasite due to the unique physiological conditions they present.
- Cysts tend to be more concentrated near the sites where muscles attach to tendons and bones. Within these muscles, the cysts are aligned longitudinally along the muscle fibers, which may enhance their structural integrity and facilitate infection upon consumption by another host.
- The deltoid muscle is commonly selected for diagnostic muscle biopsies because of its accessibility, allowing for effective identification of the parasite in clinical settings.
- Once encysted, the larvae remain infective for many years. Over time, a significant proportion of the encysted larvae become calcified and eventually die, but they can pose a risk of infection if consumed by a suitable host.
Life Cycle of Trichinella spiralis
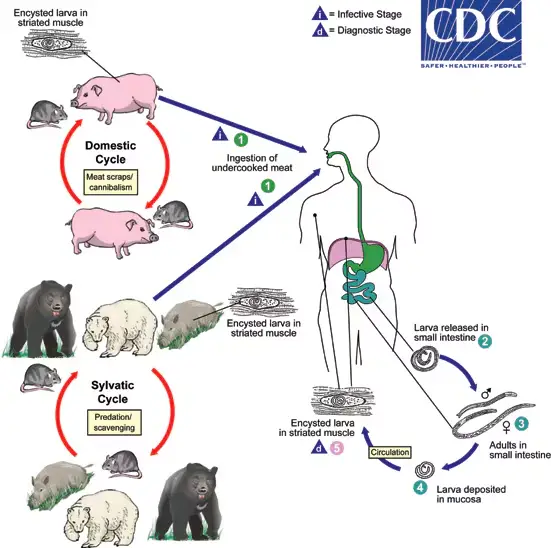
The life cycle of Trichinella spiralis is a direct cycle, which means that all developmental stages occur within a single host. However, the continuation of the species requires the transmission of infection to another host, whether of the same or a different species. This unique life cycle involves various stages and hosts, each playing a crucial role in the parasite’s survival and propagation.
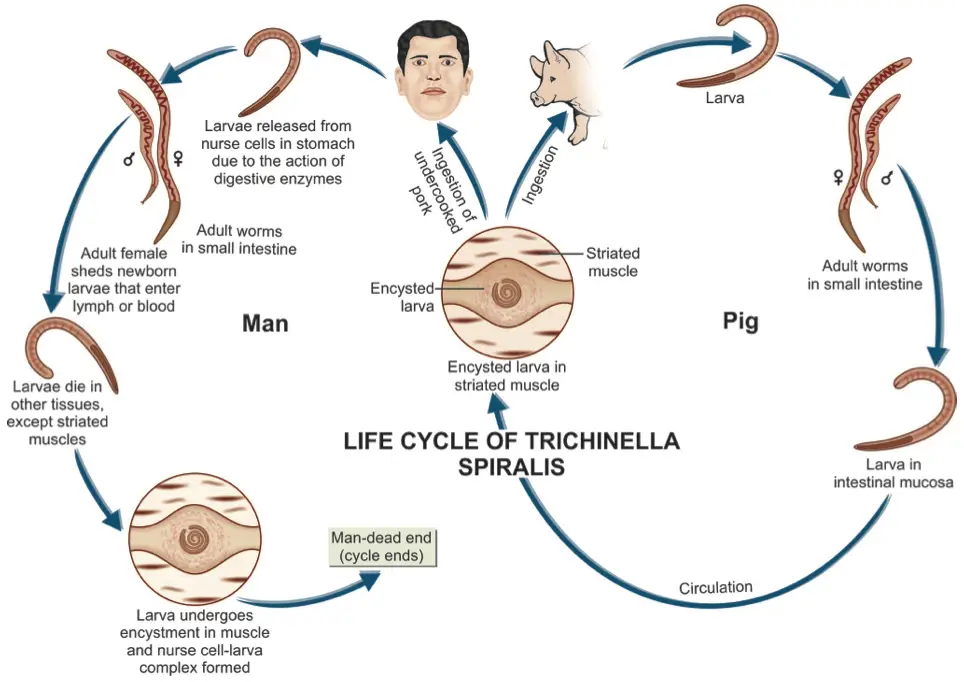
- Primary Host: The optimal host for T. spiralis is the pig, which serves as a primary reservoir for the parasite. Pigs can become infected through feeding practices, particularly when they consume untreated household garbage that may contain bits of infected meat.
- Alternate Host: Humans act as alternate hosts, but they are considered a dead-end for the parasite. This designation is due to the fact that cysts formed in human muscles are unlikely to be ingested by another host, preventing further transmission of the infection.
- Modes of Transmission:
- Infection can spread between pigs (pig-to-pig) and from rats to rats (rat-to-rat), as well as from pigs to rats (pig-to-rat). These transmission pathways highlight the importance of proper waste management and animal husbandry practices to control the spread of the parasite.
- Infective Form: The infective stage of T. spiralis is the encysted larva, which is found in the muscles of infected pigs and other animals. These encysted larvae are crucial for the lifecycle as they serve as the primary source of infection for humans and other animals.
- Infection in Humans: Humans typically acquire the infection by consuming raw or undercooked pork or inadequately processed meat products, such as sausages, that contain viable larvae. Ingesting these products without proper cooking leads to the release of larvae when the cysts are digested by gastric juices.
- Excystation and Penetration: Once ingested, the cysts are dissolved in the stomach, leading to the excystation of viable larvae in the stomach, duodenum, and jejunum. Upon release, the larvae penetrate the mucosal epithelium of the intestines.
- Development into Adults: The larvae undergo four molts, rapidly maturing into adult males and females within two days post-infection. By the fifth day, they reach sexual maturity. The male worm dies shortly after fertilization, while the female begins to release motile larvae by the sixth day of infection.
- Larval Production: Each female can produce approximately 1,000 larvae, which are released into the intestinal lymphatics or mesenteric venules. These larvae then enter the bloodstream and are circulated throughout the body.
- Tissue Distribution: Upon entering the circulation, larvae are deposited in various tissues, including muscles, the central nervous system, and other sites. While many larvae perish in these tissues, they thrive in skeletal muscle, where they undergo further development.
- Encystment in Muscle Tissue: The deposition of larvae in muscle occurs predominantly during the second week of infection, with larval development continuing over the following three to four weeks. Within 20 days, larvae encyst within muscle cells, forming structures known as nurse cells, which facilitate their survival and growth.
- Survival and Lifecycle Conclusion: The encysted larvae lie parallel to the muscle fibers and can remain viable for months or even years. In humans, the life cycle of T. spiralis concludes at this stage, as the larvae do not have another host to continue their lifecycle.
Pathogenicity and Clinical Features of Trichinella spiralis
Trichinella spiralis is a parasitic nematode responsible for the disease known as trichinosis. The pathogenicity of T. spiralis and the clinical features associated with infection can vary widely, ranging from asymptomatic cases to severe manifestations. The understanding of trichinosis requires an examination of the various stages of the parasite’s life cycle, as these stages directly correlate with the clinical symptoms experienced by the infected individual.
- Asymptomatic Infections: A significant number of individuals infected with T. spiralis may exhibit no symptoms at all. Such asymptomatic cases complicate the diagnosis and epidemiology of trichinosis, as individuals may unknowingly harbor the parasite.
- Acute Illness: Although rare, severe and potentially fatal forms of trichinosis can occur. These cases typically arise from heavy infections and are associated with serious complications.
- Life Cycle Stages and Clinical Manifestations: The clinical features of trichinosis can be categorized according to the different stages of the T. spiralis life cycle, particularly during intestinal invasion, muscle invasion, and encystation.
- Stage of Intestinal Invasion:
- This stage commences when an individual ingests raw or undercooked pork containing infective larvae.
- The larvae invade the intestinal epithelium, maturing into adult worms.
- Clinical Features: Symptoms typically manifest within 2 to 30 hours after ingestion and may include malaise, nausea, vomiting, diarrhea, and abdominal cramps. These symptoms result from the larvae penetrating the intestinal wall and the subsequent inflammatory response.
- Stage of Muscle Invasion:
- Following intestinal invasion, new infective larvae are released from the adult female worm into the bloodstream, allowing them to disseminate to various tissues, particularly striated muscle.
- Clinical Features: This stage occurs 1 to 4 weeks after initial infection. Symptoms may include fever, myalgia, periorbital edema (swelling around the eyes), weakness of affected muscles, and hemorrhagic manifestations such as subconjunctival hemorrhages and splinter hemorrhages.
- If the heart muscles are affected, myocarditis may develop, while central nervous system involvement can lead to encephalitis. A notable feature during this stage is eosinophilia, characterized by an elevated eosinophil count in the blood, which serves as a marker for parasitic infections.
- Stage of Encystation:
- The final stage involves the encystment of the larvae within striated muscle fibers, forming what are known as nurse cells.
- This stage represents a crucial adaptation that allows the larvae to survive within the host for extended periods.
- Clinical Features: In this stage, all acute symptoms generally subside as the immune system adapts to the presence of the encysted larvae. The larvae can remain viable and potentially infectious for months or even years.
- Stage of Intestinal Invasion:
Diagnosis of Trichinella spiralis
The diagnosis of trichinosis, caused by the parasitic nematode Trichinella spiralis, involves a combination of direct and indirect methods to confirm infection. Accurate diagnosis is essential for appropriate treatment and management, especially considering that the symptoms of trichinosis can overlap with those of other gastrointestinal diseases. Here, we will explore various diagnostic techniques, detailing their methodologies and relevance.
- Direct Methods: These techniques focus on identifying the parasite directly within the host’s tissues or excretions.
- Muscle Biopsy: One of the most definitive methods for diagnosis involves detecting spiral larvae in muscle tissue. The deltoid, biceps, gastrocnemius, or pectoralis muscles are typically selected for biopsy. This method allows for direct visualization of the larvae, confirming infection.
- Stool Examination: During the diarrheic stage of the disease, adult worms and larvae may be detectable in the stool. Although this method is less commonly relied upon due to the typical timing of symptoms, it can provide supportive evidence for diagnosis when present.
- Xenodiagnosis: This unique method involves feeding biopsy bits to laboratory rats. After a month, the rats are euthanized, and examination of their muscle tissues can reveal the presence of Trichinella larvae. This approach can enhance detection sensitivity compared to direct human tissue analysis.
- Indirect Methods: These techniques rely on the patient’s clinical history and serological responses to infer the presence of infection.
- Clinical History: A key initial step in diagnosis includes evaluating the patient’s history, particularly the consumption of raw or undercooked pork about two weeks prior to the onset of gastrointestinal symptoms. This contextual information is crucial, as it correlates with potential exposure to the parasite.
- Blood Examination: Eosinophilia, characterized by an increased eosinophil count (20–95%), is a common hematological finding in infected individuals. This immune response often signals the presence of parasitic infections.
- Serological Tests:
- Hypergammaglobulinemia: This condition is characterized by elevated levels of immunoglobulins, particularly serum IgE, indicating an immune response to the infection.
- Enzyme-Linked Immunosorbent Assay (ELISA): This test detects T. spiralis antibodies using specific antigens derived from infective larvae. A positive result indicates recent infection.
- Bentonite Flocculation Test and Latex Fixation Test: These methods are also employed to identify antibodies against T. spiralis, providing additional diagnostic support.
- Bachman Intradermal Test: In this test, a dilution of larval antigen is intradermally injected. A positive reaction, characterized by an erythematous wheal appearing within 15–20 minutes, suggests infection. Notably, this test can remain positive for years after the initial infection, complicating its use in determining current infection status.
- Radiological Examination: Calcified cysts can be visualized through X-ray imaging, providing another indirect indicator of T. spiralis infection. This finding typically occurs in chronic cases where encysted larvae persist in muscle tissue.
- Molecular Methods: Advances in molecular biology have introduced multiplex polymerase chain reaction (PCR) techniques for the species identification of Trichinella. This method allows for highly sensitive and specific detection of the parasite’s DNA, aiding in accurate diagnosis.
Treatment of Trichinella spiralis
The treatment of Trichinella spiralis infection, commonly known as trichinosis, varies based on the severity of the disease and the individual patient’s clinical presentation. Given that trichinosis can manifest with a spectrum of symptoms, treatment approaches must be tailored accordingly to ensure effective management of the infection and alleviation of symptoms.
- Mild Cases: In instances where the infection is asymptomatic or presents with mild symptoms, supportive care is typically sufficient. This approach may include:
- Bed Rest: Encouraging adequate rest helps the body recover.
- Analgesics: Over-the-counter pain relievers, such as acetaminophen or ibuprofen, can alleviate discomfort and muscle pain.
- Antipyretics: These medications can help reduce fever associated with the infection.
- Moderate Cases: When symptoms are more pronounced, pharmacological intervention becomes necessary. The recommended treatment options include:
- Albendazole: Administering 400 mg twice daily (BID) for eight days is effective in targeting the parasitic infection during its enteric stage.
- Mebendazole: An alternative treatment consists of 200–400 mg three times daily (TID) for three days, followed by 400 mg TID for an additional eight days. Mebendazole has similar efficacy as albendazole in managing moderate infections.
- Severe Cases: In cases where patients present with severe symptoms, particularly those involving systemic manifestations such as myocarditis or encephalitis, a more aggressive treatment regimen is warranted. This approach includes:
- Glucocorticoids: The addition of glucocorticoids, such as prednisolone, is crucial to manage inflammatory responses associated with severe infections. This combination therapy—using glucocorticoids alongside albendazole or mebendazole—can mitigate complications and improve patient outcomes.
Epidemiology of Trichinella spiralis
The epidemiology of Trichinella spiralis is a crucial area of study given its impact on public health and the transmission dynamics of trichinosis. This parasitic disease, caused primarily by T. spiralis, occurs globally and has notable geographic and demographic variations. Understanding these patterns helps in formulating effective preventive measures and public health policies.
- Global Prevalence:
- Trichinellosis occurs worldwide, with estimates suggesting approximately 10,000 cases annually. This number can vary significantly based on region, dietary habits, and agricultural practices.
- The disease typically presents in clusters, often associated with the consumption of undercooked or raw meat from infected animals, leading to localized outbreaks within communities.
- Species Diversity:
- There are nine recognized species of Trichinella, with twelve identified genotypes. Among these, Trichinella spiralis is the most prevalent species responsible for human infections. Other species, such as T. nativa, T. nelson, T. britovi, T. pseudospiralis, T. murelli, and T. papuae, have also been implicated in human cases, although to a lesser extent.
- Historical Trends:
- The epidemiological landscape of trichinosis has evolved over time. For example, the United States experienced around 400 reported cases annually in the 1940s. However, due to improved meat inspection practices and public awareness, the number of reported cases has significantly declined, averaging about 20 cases per year from 2008 to 2010.
- Demographic at Risk:
- Certain populations are at a heightened risk of trichinosis, particularly hunters and those consuming game meat. As a result, regions with substantial hunting activities, especially for wild boar or bears, see higher incidence rates due to the potential for these animals to harbor Trichinella.
- Geographic Distribution:
- The highest incidence of trichinosis is reported in China, where pork consumption rates are among the highest globally. This increased consumption heightens the risk of exposure to T. spiralis.
- In Arctic regions, wildlife such as polar bears, seals, and walrus have been identified as reservoirs for Trichinella, indicating a unique transmission dynamic in these environments.
- Emerging Trends:
- Recent consumer preferences for antibiotic-free and organic meats have inadvertently led to increased Trichinella cases in Europe. This shift emphasizes the importance of proper food handling and cooking practices, as organic farming practices can sometimes allow for less stringent control measures regarding parasite transmission.
- Prevention and Control:
- The epidemiology of Trichinella spiralis highlights the importance of public health interventions, including education about the risks associated with undercooked meat and effective farming practices that limit the exposure of pigs to Trichinella.
- Monitoring and surveillance of Trichinella in both domestic and wildlife populations are essential for managing the risk of outbreaks, especially in areas where hunting and pork consumption are prevalent.
Prophylaxis of Trichinella spiralis
Prophylaxis of Trichinella spiralis infection, which causes the disease known as trichinosis, focuses on preventing transmission through effective management practices and public health strategies. Given that the lifecycle of Trichinella spiralis is closely linked to the consumption of infected meat, particularly pork, several key measures can be employed to reduce the risk of infection.
- Proper Cooking of Meat: One of the most effective prophylactic measures is ensuring that pork and other potentially infected meats are cooked thoroughly. The following guidelines can help mitigate the risk:
- Internal Temperature: Cooking meat to an internal temperature of at least 145°F (63°C), followed by a resting time of three minutes, is crucial. This temperature is sufficient to kill encysted larvae.
- Freezing: While freezing can kill Trichinella larvae, it is only effective at specific temperatures. For example, freezing pork at -15°F (-26°C) for at least three weeks can reduce the risk of infection, but this method is less reliable than thorough cooking.
- Prevention of Feeding Practices: Another essential strategy involves addressing the feeding habits of pigs, which are the primary hosts for Trichinella spiralis:
- Avoiding Raw Garbage: The most effective method to prevent infection is to stop the practice of feeding pigs raw or inadequately processed garbage that may contain viable larvae. Implementing strict guidelines on animal feed can significantly reduce the incidence of trichinosis.
- Rodent Control: Rodents are known carriers of Trichinella, and their presence can contribute to the spread of infection:
- Extermination of Rats: Effective pest control measures should be implemented in and around pig farms. Regular extermination and preventive strategies can limit the contact between pigs and rodents, thereby reducing the likelihood of Trichinella transmission.
- Public Awareness and Education: Raising awareness about trichinosis and its transmission can empower consumers to make informed choices regarding food preparation and consumption:
- Educational Campaigns: Public health campaigns can educate individuals about the importance of cooking meat properly and recognizing the risks associated with consuming raw or undercooked pork products.
- Regulatory Measures: Governments can play a significant role in minimizing the risk of trichinosis through:
- Inspection of Meat Products: Regular inspections of meat processing facilities and enforcement of food safety regulations can ensure that infected meat does not enter the food supply.
- Surveillance Programs: Monitoring and surveillance of Trichinella in domestic and wild animal populations can help identify potential outbreaks and inform preventive strategies.
- Paniker’s Textbook of Medical Parasitology
- Rawla P, Sharma S. Trichinella spiralis Infection. [Updated 2023 Aug 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538511/
- Mahajan, M. (2021). Trichinella spiralis. Emerging Infectious Diseases, 27(12), 3155. https://doi.org/10.3201/eid2712.211230.
- Näreaho, Anu. Experimental and immunological comparison of Trichinella spiralis and Trichinella nativa.
- https://www.cdc.gov/dpdx/trichinellosis/index.html
- https://en.wikipedia.org/wiki/Trichinella_spiralis
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.