Tomato Spotted Wilt Virus, often called TSWV for short, is one of those frustrating problems that can really throw a wrench into your gardening plans or hit farmers hard. It’s not just a tomato issue either – this nasty virus has a surprisingly broad appetite, targeting everything from peppers and lettuce to popular flowers like impatiens and petunias. The real trouble comes from how it spreads: tiny insects called thrips pick up the virus as they feed and then pass it along to healthy plants as they move around. Once infected, plants often show telltale signs like bronze or dark spots on leaves, stunted growth, wilting, and oddly shaped fruit. For anyone growing their own food or managing crops, dealing with TSWV means facing potential losses and extra headaches trying to protect plants. Getting a handle on how this virus works and spotting it early is key to keeping your garden or fields thriving and productive.
What is Tomato Spotted Wilt Virus (TSWV)?
- Tomato spotted wilt virus (TSWV) is a plant-infecting virus from Tospoviridae, belongs to Orthotospovirus genus, it’s RNA based, with segmented genome
- It has 3 single-stranded RNA segments — L, M, S — coding for RNA polymerase, envelope proteins, movement and silencing suppressor proteins
- It infects 1000+ plant species, mainly tomato, capsicum, groundnut, tobacco, lettuce, ornamental plants, and several wild weeds
- Transmitted by thrips, specially Frankliniella occidentalis, only larval thrips can acquire virus, but adult spreads it forever
- Early infection shows bronze discoloration, curling young leaves, necrotic streaks on stems, tip wilting, in fruits—ring spots, distorted patches, uneven ripening
- It replicates in host cytoplasm, invades vascular tissue, suppresses RNA silencing, blocks plant immunity, causes systemic spread
- It spreads rapidly in greenhouses or open fields where thrips population is high and weeds are unmanaged
- Causes heavy yield loss, sometimes 80–100%, especially in tomato or pepper cultivation, makes fruit unmarketable
- No treatment available post-infection, it’s managed by integrated strategies—
- – Use resistant cultivars (like Sw-5 gene tomatoes)
- – Control thrips with insecticides or biological agents
- – Remove weed hosts and infected plants
- – Use reflective mulches to deter thrips
- Some thrips-adapted TSWV strains can infect Sw-5 cultivars, so it’s not always fully effective
- ELISA, RT-PCR, LAMP assays commonly used for TSWV detection in field or lab
Tomato spotted wilt virus insect vector
- Insect group – it’s transmitted only by thrips (tiny insects under order Thysanoptera), no other insect vectors confirmed
- Transmission type – it uses circulative‑propagative pathway inside vector, virus multiplies within the thrips body and reaches salivary glands before transmission
- Major vector – Frankliniella occidentalis (western flower thrips) transmits most cases globally, highly invasive, common in greenhouse setups
- Other vectors – it includes Thrips tabaci (onion thrips), Frankliniella fusca (tobacco thrips), Scirtothrips dorsalis (chilli thrips), also some regional species
- Infection stage – only larval thrips can acquire virus when feeding on infected plants, but then it, this vector, keeps spreading virus whole life
- Infectious adults – once it’s acquired, adult thrips don’t lose infectivity, they transmit virus for life, even if virus no longer present in plant
- Spread influence – it spreads faster if thrips population high, if host plants or weeds left unmanaged, or if insecticides miss larval stages
- Biological notes – inside thrips, it’s viral load regulated by insect immune responses like Toll pathway, which controls how much virus accumulates
- Ecological fit – vector species distribution varies by climate—F. occidentalis found everywhere, others like F. schultzei more tropical
Tomato Spotted Wilt Virus (TSWV) Classification and Taxonomy
- Realm – it belongs to Riboviria, the realm of RNA viruses encoding RNA-dependent RNA polymerase, it encompasses many viruses of plants, animals and humans.
- Kingdom – Orthornavirae, grouping viruses with RNA genomes dependent on RdRp for replication, negative‑strand included.
- Phylum – Negarnaviricota, negative-sense single‑stranded RNA viruses infecting eukaryotes.
- Subphylum – Polyploviricotina, a further division within negative-sense viral phyla.
- Class – Bunyaviricetes, the class formerly Bunyaviridae members but reclassified under Bunyavirales.
- Order – Elliovirales, the order containing plant‑infecting tospoviruses.
- Family – Tospoviridae, the family that consists exclusively of tospoviruses infecting plants and vectored by arthropods.
- Genus – Orthotospovirus, named for the first species Tomato spotted wilt virus, now encompassing ~28 species with similar genome structure and thrips transmission
- Species – Orthotospovirus tomatomaculae (TSWV), the formal species name, symbolic type of the genus, defined by <90 % nucleocapsid homology cutoff and vector specificity
- Baltimore class – Group V: negative-sense single-stranded RNA viruses, using ambisense coding on M and S segments .
- Genome structure – tripartite linear RNA: L (~8.9 kb) encodes RdRp; M (~5.4 kb) encodes glycoproteins and NSm; S (~2.9 kb) encodes nucleocapsid (N) and NSs suppressor .
- Historical note – first recorded in Australia ~1915, formally described in 1930; genus named after it; it remained the only recognized tospovirus until genetic screening expanded known species in 1990s
Tomato Spotted Wilt Virus (TSWV) 3D Model
Structure of Tomato Spotted Wilt Virus (TSWV)
- Shape – it’s spherical to pleomorphic, 80–120 nm wide, covered in lipid bilayer taken from host membrane, sometimes irregular
- Envelope glycoproteins – it carries two major glycoproteins, Gn and Gc, both embedded in outer membrane, they help in vector infection and virion assembly
- Internal structure – inside it, there’s ribonucleoprotein (RNP) complex, each RNA coated by nucleocapsid protein (N), forming loose helical coils
- Genome – it holds three single-stranded RNA segments—
- – L (~8.9 kb): encodes L protein (RNA-dependent RNA polymerase)
- – M (~5.4 kb): encodes Gn, Gc, and NSm (movement protein)
- – S (~2.9 kb): encodes N protein and NSs (silencing suppressor)
- N protein – nucleocapsid protein wraps RNA loosely, it forms ring-like trimer units, with arms folding over RNA groove, allows flexibility
- Polymerase – L protein (RdRp) remains associated with RNP, not freely floating, replicates and transcribes viral genome once inside host
- NSm – non-structural movement protein, it creates tubules that span plant cell walls, moves viral RNA-protein complex across infected tissue
- Localization – it’s seen in cytoplasm, often within ER lumen or associated with viral inclusion bodies, which look like fibrous masses
- Functionality – each part plays role — envelope helps thrips infection, N binds RNA, NSm spreads it, L replicates it, NSs suppresses host defense
- Visibility – under EM, it appears like round or ovoid particles with spiky projections due to Gn/Gc, internal dark core from RNP
Genomic Organization of Tomato Spotted Wilt Virus (TSWV)
It has a tripartite, single‐stranded, negative-sense RNA genome named L, M, S segments, each packaged in RNP form and enveloped in virion
- L segment – ~8.9 kb long, negative-sense polarity, encodes a single large open reading frame in viral‑complementary sense for the RNA-dependent RNA polymerase (RdRp, ~331‑kDa)
- M segment – ~4.8–5.4 kb, ambisense arrangement:
- – one ORF in viral sense encodes movement protein NSm (~33.6 kDa)
- – second ORF in viral-complementary sense encodes glycoprotein precursor (~127 kDa) processed to Gn (~78 kDa) and Gc (~58 kDa).
- S segment – ~2.9 kb, ambisense gene layout:
- – viral-complementary strand encodes nucleocapsid protein N (~28.8 kDa)
- – viral-sense encodes NSs (~52 kDa) which suppresses RNA silencing in host.
- Ambisense organization – M and S segments contain two non-overlapping ORFs separated by AU-rich intergenic region, one gene in each polarity, allowing timely expression.
- Terminal non-coding regions – both 5′ and 3′ ends of each segment are complementary, forming a panhandle secondary structure; minimal 5′ (~17‑nt) and ~50‑nt 3′ UTR sequences are essential for replication initiation.
- Subgenomic RNAs – M and S segments produce two subgenomic mRNAs each (from vc and v-sense), whereas L segment expresses full-length mRNA without subgenomic forms.
- RNP composition – genomic RNAs are always coated by multiple N protein monomers forming ribonucleoprotein complexes, each also associated with RdRp; naked RNA is non-infectious.
- Small interfering RNA hotspots – RNA‑seq studies show differential vsiRNA accumulation across segments; in resistant Sw‑5(+) tomato, more vsiRNAs from L RNA, whereas in susceptible Sw‑5(−), S RNA dominates hotspots.
- Expression summary – L encodes RdRp, M encodes NSm and glycoproteins Gn/Gc, S encodes N and NSs, all arranged in negative or ambisense orientation for regulated viral gene expression
Gene Expression of Tomato Spotted Wilt Virus (TSWV)
- Expression style – it uses ambisense coding in M and S segments, while L segment expresses from negative-sense strand only
- L segment expression – it transcribes full-length mRNA from vc-sense only, encodes the RNA-dependent RNA polymerase (RdRp), known as L protein
- M segment expression – it produces two subgenomic mRNAs—
- – from viral-complementary (vc) strand: glycoprotein precursor (Gn/Gc)
- – from viral-sense strand: NSm movement protein
- S segment expression – also ambisense, gives rise to—
- – vc strand: nucleocapsid (N) protein
- – v strand: NSs protein, which suppresses RNA silencing
- Subgenomic RNAs – it generates subgenomic RNAs for all ambisense genes, allowing staggered and regulated gene expression
- Intergenic region – it lies between the two ORFs in M and S segments, AU-rich, forms hairpin that halts transcription and separates gene directions
- Enhancer role – this intergenic hairpin helps mRNA stability and translation, even though it lacks poly-A tail
- Silencing suppression – NSs from S segment interferes with host defense by binding siRNAs, enabling the virus to express its genome freely
- Cap-snatching – since some mRNAs are uncapped, it may hijack host mRNA caps or use RNA structures for translation, but that still debated
- Timing – first it expresses vc-sense genes (N, NSm), then v-sense genes (NSs, glycoproteins), after sufficient replication has begun
- Defense resistance – NSm protein triggers Sw-5 resistance gene response in tomato, thus it becomes a key in host-pathogen interaction
- Expression in host – it varies by cultivar, in resistant plants viral genes expressed weakly, in susceptible ones they dominate early response
- Vector expression – even in thrips, its genes express in gut and salivary tissue, aiding virus propagation and lifelong transmission
Replication of Tomato Spotted Wilt Virus (TSWV)
In Plants;
- Entry – it enters plant cells mostly through physical wounds (effraction), or through feeding punctures by thrips, then spreads between cells via plasmodesmata
- Initial transcription – once inside cytoplasm, it, this virus uses its own RNA-dependent RNA polymerase (L protein) to transcribe viral mRNAs from encapsidated genomic RNA
- Protein synthesis – host ribosomes translate these mRNAs to produce viral proteins including nucleocapsid protein (N), movement protein (NSm), glycoproteins, and silencing suppressor (NSs)
- Replication trigger – it begins replication only after enough N protein is available, so new genomes and antigenomes can be encapsidated into ribonucleoprotein (RNP) complexes
- RNP formation – those RNPs, made of genomic RNA + N protein + RdRp, are biologically active and serve both for translation and as templates for more transcription
- Cell-to-cell movement – it moves into neighboring cells as RNPs pass through plasmodesmata, aided by NSm protein, no need for full virion at this stage
- Systemic infection – after spreading cell-to-cell, it reaches phloem and moves long-distance, infecting entire plant gradually
- Alternative route – in some cases, it assembles full virions that bud from the internal membrane or plasma membrane, and these then infect nearby cells or tissues
- Membrane exit – it exits cell by budding, using host-derived lipid bilayer to form enveloped virions with glycoproteins Gn and Gc on surface
- Outcome – once systemic, it, this infection leads to symptom expression like wilting, necrosis, and fruit deformation, depending on host and viral strain
In Insects;
- Attachment – it binds to receptors on insect host cells using Gn and Gc glycoproteins present on viral envelope, this allows specific recognition by vector species
- Fusion – after attachment, its envelope fuses with membrane of host cell vesicles, then ribonucleocapsid gets released into the cytoplasm
- Cytoplasmic location – entire replication cycle happens in cytoplasm, it doesn’t enter nucleus at any stage
- Transcription – its RNA-dependent RNA polymerase begins transcribing the viral RNA segments into mRNAs directly from incoming ribonucleocapsid
- Protein production – host ribosomes translate these mRNAs to produce viral proteins, especially nucleoprotein (N), glycoproteins, and L protein itself
- Replication trigger – once enough N protein is synthesized, it encapsidates the newly made genomes and antigenomes to form new ribonucleoprotein complexes
- RNP formation – this new generation of RNPs becomes biologically active, and some also serve as templates for further replication rounds
- Virion assembly – new virions are formed when RNPs bud through internal membranes, wrapping themselves in lipid envelope containing Gn/Gc
- Exit – those virions exit by budding through the insect cell membrane, it’s how they get packaged for next transmission cycle
- Outcome – this lets insect remain viruliferous for life, even if virus not actively replicating later, making it efficient vector for TSWV
Tomato spotted wilt virus symptoms
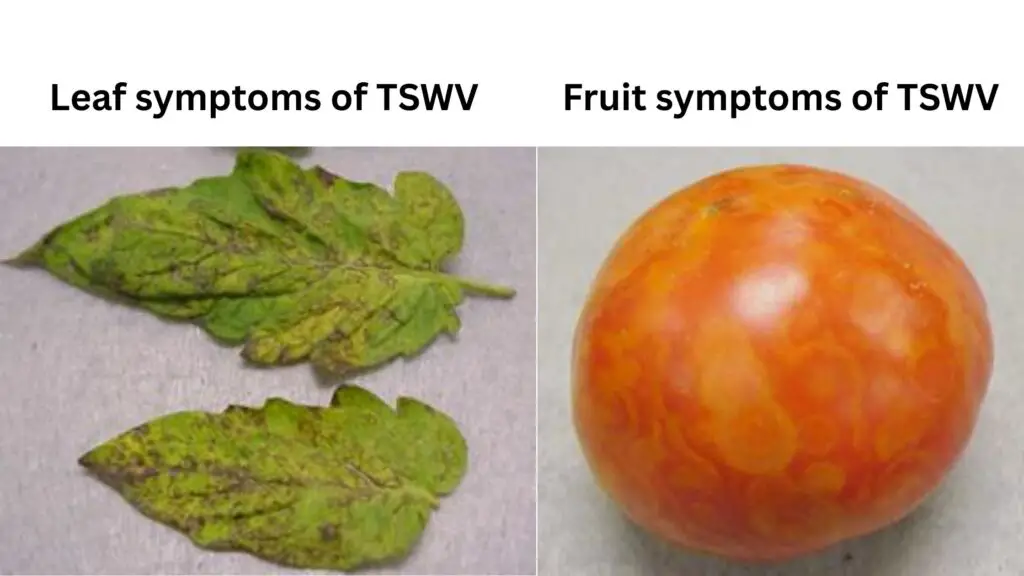
now correcting it exactly by your rules:
- Stunting – it causes reduced growth, often severe in young seedlings, they look weak, sometimes form tight rosettes, growth stalls early
- Leaf bronzing – this symptom shows as bronze or purplish color on top side of young leaves, it makes them curl downward or hang limp
- Necrotic spots – it begins with dark flecks on leaves, later they turn to brown-black patches, sometimes form ring spots with pale halos
- Leaf distortion – it causes leaves to curl, twist abnormally, cup inward, sometimes veins turn dark or die along their length
- Tip dieback – growing tips wilt first, then dry out, upper parts collapse gradually, whole plant may wilt if infection spreads fast
- Stem lesions – stems show dark streaks near nodes or joints, it may crack or split later, making plant look severely damaged
- Fruit ringspots – fruits get yellow or brown concentric circles, like a target pattern, very typical of this infection
- Fruit deformation – tomato and pepper fruits often shrink, become bumpy, get cracks, uneven color, mostly unusable for market
- Host variation – symptoms differ slightly by plant type, tomato shows leaf spots and fruit rings, peanut mostly gets stunting and yellowing
- Combined effect – This virus, creates a combo of leaf bronzing, ring patterns, stem darkening and stunting that’s hard to miss
Tomato spotted wilt virus life cycle
It cycles between plant hosts and insect vectors, involving virus entry in both plus replication and spread across tissues and organisms
Acquisition in larva – it’s taken up when thrips larvae feed on infected plants; only larvae can pick it up, after which adults transmit for life.
Thrips infection – it attaches via Gn/Gc glycoproteins to insect gut receptors, fuses with vesicle membrane, releases ribonucleocapsid into cytoplasm for transcription and replication.
Insect replication – it replicates in the cytoplasm; viral polymerase transcribes genomic RNAs, proteins like N, NSm, NSs and glycoproteins are synthesized; replication begins when N protein levels suffice to form RNPs, which bud off enveloped virions via insect cell membranes.
Adult vector transmissibility – it persists in adult thrips that acquired it as larvae; these remain efficient lifelong vectors but do not pass it to eggs or larvae of next generation.
Inoculation of plant – adult thrips feed on host plants and transmit virus into cells by puncturing, injection into cytoplasm through wounds
Plant replication – it enters plant cells via effraction or plasmodesmata, its polymerase transcribes viral mRNAs from encapsidated RNA, proteins produced lead to RNP formation once sufficient N present; RNPs then move cell-to-cell via plasmodesmata aided by NSm, or bud off as full virions at plasma/ER membranes to infect neighboring tissue.
Systemic spread – it spreads through plasmodesmata into vascular tissue, later systemic infection develops in days to weeks; infected tissue becomes source for future acquisition by thrips larvae.
Life cycle time‑frame – thrips develop from egg to adult in about 10–18 days, larval stage of 1‑3 days critical for virus acquisition, adult feeds and transmits subsequently; plant symptoms develop about 1–3 weeks after inoculation depending on age and environment.
Transmission cycle summarised – it, this virus circulates: infected plant → larval thrips acquisition → replication in insect → adult thrips transmission → plant infection → plant spread → new acquisition
Factors influencing infection and spread of Tomato spotted wilt virus
Here are the Factors that influence the infection and spread of Tomato spotted wilt virus;
- Inoculum presence – it spreads more when infected weed hosts are present nearby, these act as early-season virus sources
- Vector density – when thrips population is high, especially in spring or dry weather, the virus spreads rapidly to crops
- Environmental conditions – it thrives in warm, dry periods, rain sometimes suppresses thrips, but heat makes outbreaks more severe
- Planting date – if crop is planted during peak thrips emergence, infection rate shoots up, older plants resist it slightly better
- Host availability – it spreads faster when susceptible crops like tomato or pepper are grown near thrips-attracting weeds
- Insect behavior – infected plants attract more thrips due to reduced repellents, so this virus manipulates host smell to enhance spread
- Virus-vector fitness – it makes thrips live longer, lay more eggs, increasing the time and chance for it to be passed around
- Cultivar susceptibility – crops without Sw‑5 or Tsw gene fall victim fast, while resistant ones slow down spread, though not always fully
- Landscape pattern – it moves quicker in patchy fields, or near weed-infested plots, where thrips can hop from plant to plant
- Insect control – if thrips aren’t managed early, it spreads before symptoms show up, contact insecticides work best if used on time
Management of Tomato Spotted Wilt Virus (TSWV) Disease
Here’s an improved Disease Management summary for TSWV, now using web-supported info and formatted per your style:
- Home garden focus – it’s about avoiding infected transplants, destroying symptomatic plants, and lightly using botanical methods rather than chemicals
- Early prevention – it relies heavily on integrated tactics used before or immediately after transplanting – insecticides must be preventive, not curative
- Resistant varieties – those carrying Sw‑5 gene (e.g. ‘Jimbo’, ‘Southern Star’, ‘Amelia’) offer partial protection though resistance‑breaking strains have emerged
- Thrips control – it needs weekly scouting; action threshold ~5 thrips per bloom; control larval stages via insecticides like imidacloprid at transplant and early weeks
- Reflective mulch – UV‑reflective ground cover reduces adult thrips landing, hence slows virus spread especially early season
- Weed and greenhouse hygiene – it’s essential to remove virus‑hosting weeds and avoid growing ornamentals near vegetables; sanitize soil and seedlings
- Selective insecticides – spinosyns (e.g. spinetoram) most effective against thrips, rotate modes-of-action (imidacloprid, cyantraniliprole, flonicamid) to manage resistance
- Botanical/biological options – neem oil, insecticidal soaps or oils may suppress thrips in home gardens; entomopathogenic fungi (Beauveria, Metarhizium) and predatory bugs (Orius, Geocoris) offer biological control options
- System approach – it uses multiple practices together: resistant plants, early insecticide, reflective mulch, weed control, scouting—no single method suffices alone
- Timing matters – thrips transmit virus within days after transplant; insecticidal drench at planting highly beneficial; contact sprays early weeks maximize protection
Cultural Practices for Tomato Spotted Wilt Virus (TSWV)
Implementing effective cultural practices is crucial for managing Tomato Spotted Wilt Virus (TSWV) in tomato crops. Key strategies include:
- Crop rotation – it rotates susceptible crops with non‑hosts to break virus cycle and reduce thrips breeding grounds, this suppresses future inoculum build‑up.
- Weed management – it removes weed hosts that harbor TSWV and thrips in and around fields or greenhouses, reducing primary spread
- Reflective mulch – it uses UV‑reflective silver or metallic mulch in early season, reflecting light deters adult thrips landing and lowers virus transmission.
- Sanitation and plant removal – it involves promptly removing and destroying all symptomatic and infected plants, preventing secondary spread.
- Use virus‑free transplants – it sources clean seedlings from certified nurseries, ensures no early infection is introduced into the crop.
- Floating row covers – it covers young plants with row covers (e.g. radicchio example) to shield them from thrips, drastically reducing TSWV incidence (<1 %)
- Optimal planting date – it avoids early planting when thrips pressure is highest; delayed planting (after peak migration) reduces disease risk in peanut and tomato systems.
- Fertility management – it avoids excess nitrogen fertilization which increases thrips attraction and virus risk by boosting plant amino acids and pest reproduction.
- Spacing and airflow – it ensures adequate plant spacing to improve air circulation and reduce thrips habitat, though direct data limited, standard cultural hygiene applies.
Tomato spotted wilt virus resistant varieties
Here are examples of tomato and pepper varieties with resistance to Tomato spotted wilt virus (TSWV):
- Tomato cultivars with Sw‑5b
- LA 3667, Paronset, G17‑60 carry Sw‑5b in homozygous form, shown resistant to TSWV in controlled inoculation tests.
- IVF3545 is heterozygous Sw‑5b R/S, also exhibits resistance under lab and greenhouse conditions
- Many commercial tomatoes carry Sw‑5b locus, though few are Sw‑5b R homozygotes, eg only ANX among 46 tested was heterozygous; most lacked resistance altogether.
- Wild relative resources containing resistance genes
- Lines from Lycopersicon peruvianum (eg PI 126935, PI 126944, CIAPAN 16, PE‑18, CIAPAN 17) possess natural resistance, some used to introgress Sw‑6 or Sw‑7 genes.
- Sw‑6 and Sw‑7 gene lines
- Sw‑6 from L. peruvianum accessions provides partial, isolate-specific resistance, useful as supplementary R gene.
- Sw‑7 from L. chilense accession LA 1938 confers field resistance to diverse TSWV strains including those breaking Sw‑5, used in Florida and South Africa trials.
- Pepper varieties with Tsw gene
- Tsw gene in pepper (Capsicum chinense derived) provides dominant resistance to TSWV in pepper, although resistance‑breaking strains may emerge.
- Grafted tolerant ecotype
FAQ
What is Tomato Spotted Wilt Virus (TSWV) and what are its general symptoms?
Tomato Spotted Wilt Virus (TSWV) is a significant plant-infecting virus belonging to the Orthotospovirus genus within the Tospoviridae family. It is an RNA-based virus with a segmented genome and is considered one of the most economically devastating plant viruses globally, causing over $1 billion in annual economic impact. TSWV has an exceptionally broad host range, infecting more than 1,000 plant species across 85 families, including crucial agricultural and horticultural crops like tomatoes, peppers, peanuts, tobacco, lettuce, and various ornamental plants and weeds.
Symptoms of TSWV are highly variable depending on the host plant, the specific virus isolate, and environmental factors such as temperature. However, common symptoms across infected plants include:
Stunting: Reduced overall plant growth, often severe in young seedlings, leading to weak, sometimes tight, rosette-like growth.
Leaf Bronzing/Purpling: A bronze or purplish discoloration on the upper side of young leaves, which may also cause them to curl downwards.
Necrotic Spots and Lesions: Dark flecks that develop into brown-black patches, often forming characteristic ring spots with pale halos on leaves. In severe cases, entire leaves may bronze, roll, and wilt.
Leaf Distortion: Leaves may curl, twist abnormally, or cup inwards, with veins sometimes darkening or dying along their length.
Tip Dieback: Growing tips may wilt and dry out, leading to the collapse of upper plant parts, and potentially the entire plant if the infection spreads rapidly. Seedlings can exhibit a distinctive “shepherd’s crook” appearance before dying.
Stem Lesions: Dark streaks can appear on stems near nodes or joints, which may later crack or split, causing severe damage.
Fruit Symptoms: Infected fruits, especially tomatoes and peppers, often show yellow or brown concentric rings (target patterns), become bumpy, deformed, shrink, develop cracks, and ripen unevenly, making them unmarketable.
Early infection can lead to total crop loss, while later infections typically result in significant reductions in quality and marketable yield.
How is TSWV transmitted, and what are the characteristics of its vectors?
TSWV is exclusively transmitted by tiny insects called thrips, belonging to the order Thysanoptera. It is not transmitted through seeds. The transmission process is circulative-propagative, meaning the virus multiplies within the thrips’ body before reaching their salivary glands, making them persistent vectors.
Key aspects of TSWV transmission and its vectors include:
Primary Vectors: The most significant global vector is the Western Flower Thrips (Frankliniella occidentalis), common in both open fields and greenhouses. Other important vectors include the Tobacco Thrips (Frankliniella fusca) and Onion Thrips (Thrips tabaci), and some regional species like Chilli Thrips (Scirtothrips dorsalis).
Acquisition Stage: Crucially, only larval-stage thrips can acquire the virus when feeding on infected plants. Adult thrips cannot acquire TSWV because their midgut barrier prevents infection.
Transmission Stage: Once acquired as larvae, thrips remain infective and can transmit the virus throughout their adult lives (30-45 days). They do not lose infectivity.
Inoculation: Adult thrips transmit the virus to healthy plants by puncturing plant tissue with their mandibles and ingesting cellular fluid, releasing virus particles through their saliva. Inoculation can occur in as little as five minutes of feeding.
Life Cycle and Spread: Thrips have a rapid developmental rate (egg to adult in 7.5–13 days, depending on temperature), contributing to quick virus spread. Eggs are laid inside plant tissues, and larvae often remain in protected areas, making them difficult to target with contact insecticides. After two larval stages, thrips move to soil or leaf litter for an inactive pupal stage. Warmer temperatures accelerate their development and can lead to more severe outbreaks.
Movement: Thrips are tiny and can be carried by wind from surrounding areas or on clothing, facilitating widespread virus dissemination.
The effectiveness of RNAi against thrips can vary significantly between species and at different life stages, posing additional challenges for management.
What are the current conventional strategies for managing TSWV in crops?
Current management strategies for TSWV focus on an integrated disease management approach due to the virus’s wide host range, the thrips’ ability to develop pesticide resistance, and the fact that infected plants cannot be cured. These strategies aim to prevent infection and reduce virus spread:
Resistant Cultivars:
Growing TSWV-resistant plant varieties is often the primary tactic. Many tomato cultivars carry the Sw-5 gene, and pepper cultivars the Tsw gene, which confer resistance, often through a hypersensitive response that causes localized cell death to limit viral spread.
However, TSWV isolates have shown the ability to overcome these resistance genes (e.g., Sw-5 resistance-breaking strains have been reported in the US, Australia, and Spain), necessitating a multi-faceted approach.
Resistance genes are typically expressed in foliage but not in flowers and fruit, meaning thrips feeding on flowers of resistant plants can still transmit the virus to fruit, though not to the foliage unless it’s a resistance-breaking strain.
Thrips Management (Vector Control):
Insecticides: Pesticide application targets thrips populations. Spinosad is cited as effective against F. occidentalis. Systemic neonicotinoid insecticides like imidacloprid can interfere with tobacco thrips feeding. However, thrips rapidly develop resistance to broad-spectrum insecticides, and their small size and tendency to hide make contact pesticides less effective. Overuse and lack of insecticide rotation contribute to resistance development.
Reflective Mulches: Using metallic-reflective mulches can deter airborne thrips by interfering with their perception, reducing thrips landing and TSWV incidence. While effective, they can reduce soil temperature and delay plant growth early in the season.
Biological Control: Introducing natural predators, such as minute pirate bugs (Orius genus) and big-eyed bugs (Geocoris punctipes), can reduce thrips populations, especially in controlled environments. However, their direct impact on TSWV incidence is less studied, and they can be vulnerable to insecticides.
Greenhouse Exclusion: Installing fine mesh screening (e.g., 400 mesh) on air intakes and vents can physically block thrips entry. Double-door entries add further protection.
Cultural Practices and Sanitation:
Weed Management: Controlling weeds both within and around crop fields and greenhouses is crucial, as many weed species serve as reservoirs for both TSWV and thrips, allowing the virus to overwinter.
Removal of Infected Plants: Promptly removing and destroying symptomatic and infected plants prevents them from serving as ongoing sources of the virus for new infections.
Virus-Free Transplants: Starting with healthy, virus-free plant material from certified nurseries is essential to prevent early introduction of TSWV. Inspect all incoming plant material for symptoms and thrips. Avoid growing vegetable starts in the same greenhouse as ornamentals, as many ornamentals are TSWV hosts, some even asymptomatic carriers.
Crop Rotation: Rotating susceptible crops with non-hosts can help break the virus cycle.
Planting Date: Adjusting planting dates to avoid periods of peak thrips emergence can reduce infection rates.
Monitoring: Regularly monitoring for thrips using yellow sticky cards just above the crop canopy helps track population levels and inform management decisions.
Post-Harvest Sanitation: Promptly removing and destroying all old crop material after harvest, followed by allowing ground to lay fallow, helps break the thrips life cycle.
An integrated approach combining these strategies is generally most effective, as no single method provides foolproof protection against TSWV.
What is RNA interference (RNAi) and how does it relate to plant defense against viruses?
RNA interference (RNAi) is a natural genetic regulatory mechanism found across plants, animals, and fungi. It involves the use of small RNA (sRNA) molecules, derived from double-stranded RNA (dsRNA), to silence gene expression. This process plays a vital role in various biological functions, including development, transposon silencing, and crucially, defense against invading pathogens like viruses.
In the context of plant-virus interactions:
Mechanism: Plants utilize RNAi as an immune response against invading viruses. When a virus with a single-stranded RNA (ssRNA) genome (like TSWV) replicates, it forms double-stranded RNA intermediates through the action of its RNA-dependent RNA polymerase (RdRp). Alternatively, secondary stem-loop structures within the viral genome can also form dsRNA.
siRNA Production: These dsRNA forms serve as substrates for an enzyme called Dicer, which cleaves them into small interfering RNA (siRNA) molecules, typically 20-25 nucleotides long.
Gene Silencing: These siRNAs then guide the RNA-induced silencing complex (RISC) to target and degrade complementary messenger RNA (mRNA) molecules from the virus. By silencing the expression of critical viral genes, RNAi effectively impedes viral infection and replication.
Viral Counter-Defense: Plant viruses, including TSWV, have evolved counter-mechanisms to subvert or suppress the host’s RNAi response. TSWV’s non-structural silencing suppressor (NSs) protein, for example, sequesters siRNA molecules, preventing them from being loaded onto RISC, and can also inhibit the processing of large dsRNA precursors into siRNAs. NSs also plays a role in attracting thrips to infected plants by suppressing terpene synthesis, which would otherwise repel the insects, and is essential for persistent infection in thrips.
Understanding RNAi pathways and viral silencing suppressors is critical for developing new, targeted strategies for viral disease management.
How has transgenic RNAi been used to create TSWV-resistant plants?
Transgenic RNA interference (RNAi) has proven to be a valuable approach for engineering plants with resistance to specific viruses, including TSWV. This method involves genetically modifying plants to express specific dsRNA sequences that trigger an RNAi response against the virus.
Here’s how it works and its application for TSWV:
Engineering Resistance: Researchers typically engineer plants to contain an expression cassette with an inverted repeat sequence homologous to a critical viral gene. This sequence is designed to be expressed in both sense and antisense forms, linked by a spacer (often an intron to enhance processing).
Hairpin RNA (hpRNA) Formation: When this construct is transcribed in the host plant, it produces a long hairpin RNA (hpRNA). The sense and antisense sequences anneal together to form dsRNA, with the spacer forming a hinge loop.
siRNA Generation and Silencing: This hpRNA acts as a substrate for the plant’s RNAi machinery, leading to the production of siRNAs that are complementary to the targeted viral gene. These siRNAs then guide the silencing complex to degrade the corresponding viral mRNA, thereby inhibiting viral replication and infection.
Early TSWV Resistance Research: The first instance of transgenic RNAi-induced TSWV resistance was observed somewhat inadvertently. Tobacco plants engineered to express TSWV’s nucleocapsid (N) protein showed resistance, which was later attributed not to the N protein itself, but to the RNA homologous to the N gene sequence triggering an RNAi response.
Targeting Viral Genes: Subsequent research has thoroughly investigated RNAi for TSWV resistance. Studies showed that targeting the non-structural movement protein (NSm) or, most effectively, the nucleocapsid (N) gene of TSWV resulted in significant resistance. A minimum sequence length (e.g., 110 bp for the N gene) was found necessary to trigger effective RNAi.
Cross-Protective Resistance: Transgenic RNAi can also provide resistance to multiple viruses. Chimeric constructs combining N gene sequences from TSWV and other tospoviruses (e.g., Groundnut ringspot virus, Tomato chlorotic spot virus) resulted in resistance to all these viruses. Similarly, combining TSWV’s N gene sequence with a gene from a different virus genus (e.g., Begomovirus) provided cross-protective resistance. Targeting highly conserved genes across virus species (like the RdRp gene in tospoviruses) can also induce broad-spectrum resistance.
Despite its success, challenges remain, such as the potential for viruses to overcome resistance through mutations, and public acceptance/regulatory hurdles for genetically modified crops.
How can RNAi be used to control thrips vectors directly?
RNA interference (RNAi) also holds significant potential as a bio-insecticide to directly control insect pests like thrips by silencing their essential genes. While the RNAi mechanism functions in most insects, the methods of delivering dsRNA to pest species outside of a laboratory setting present practical challenges.
Here’s how RNAi is being explored for thrips control:
Mechanism in Insects: Similar to plants, insects utilize dsRNA as a substrate to initiate an RNAi response, leading to the silencing of homologous target gene sequences. RNAi also plays a role in insect antiviral defense.
Delivery Methods:
Microinjection: Direct microinjection of dsRNA into insects has shown success in reducing gene expression, survival, and fertility (e.g., targeting the V-ATPase-B gene in F. occidentalis). However, this is impractical for field applications.
Feeding on dsRNA-containing media: Thrips feeding on media or plant tissues containing dsRNA has demonstrated effectiveness. For instance, feeding F. occidentalis on bacteria expressing dsRNA against an essential alpha-tubulin gene resulted in significant mortality, especially in larvae. Bioassay systems using leaf discs soaked in dsRNA solutions have also induced mortality and gene knockdown in thrips.
Transgenic Plants: A common approach is to use transgenic plants that constitutively express a dsRNA sequence targeting a critical insect gene. When thrips feed on these plants, they ingest the dsRNA, which then, in theory, spreads from their midgut to initiate an RNAi response, potentially leading to mortality or increased susceptibility to other control methods.
Topical Application (Sprays): More recently, topical application of dsRNA as a spray solution directly onto plant tissue has been explored. Studies have shown that thrips feeding on capsicum leaves sprayed with dsRNA targeting an aquaporin gene (F. occidentalis) resulted in increased thrips mortality and reduced gene expression. This method bypasses the need for transgenic plants and is more practical for field use.
Targeting Essential Genes: The goal is to silence genes critical for thrips survival, reproduction, or susceptibility to other treatments. Examples include V-ATPase-B, alpha-tubulin, and aquaporin genes.
Challenges: Effective RNAi in insects is highly variable between species, tissues, and life stages. Additionally, dsRNA can degrade in artificial diets, but feeding on plants that have absorbed dsRNA (e.g., via petiole-dip or spray) has shown promise. Off-target effects on beneficial insects (like pollinators) must be carefully assessed to ensure the specificity of chosen dsRNA sequences.
Despite challenges, direct RNAi-based control of thrips vectors represents a promising novel bio-insecticide strategy.
What are the advantages and limitations of topical application of RNAi for TSWV management?
Topical or exogenous application of RNAi triggers (dsRNA or siRNAs) is an emerging and promising strategy for crop protection against viruses and insect pests, offering several advantages over traditional methods, but it also comes with notable limitations.
Advantages of Topical RNAi:
Avoids Transgenics: It eliminates the need for genetically modified (transgenic) plants, addressing concerns about consumer acceptance and complex regulatory hurdles associated with GMOs.
Ease of Use: Foliar application, typically through spraying, is a simple and practical method for delivering the RNAi triggers to plants.
Targeted Action: dsRNA sequences can be precisely designed to target critical viral genes (e.g., TSWV’s N gene) or essential insect genes (e.g., thrips’ aquaporin), leading to sequence-specific RNAi responses.
Environmental Profile: Compared to traditional chemical pesticides, topical RNAi applications show attractive properties such as:
Zero or reduced crop residues: dsRNA is a natural molecule that is expected to degrade rapidly in the environment.
Minimal off-target impacts: With careful design, dsRNA can be highly specific to the pest or pathogen, reducing harm to beneficial insects, wildlife, and the environment.
Low risk for resistance: The natural, gene-silencing mechanism may pose a lower risk for rapid resistance development compared to chemical insecticides, though viruses can still mutate to overcome specific RNAi targets.
Broad Applicability: This approach has shown success against various plant viruses (e.g., pepper mild mottle virus, Cymbidium mosaic virus, sugarcane mosaic virus, cucumber mosaic virus) and insect pests (e.g., Colorado potato beetle, thrips).
Efficacy Against Insect-Transmitted Viruses: It has been shown effective even when the virus is introduced by an insect vector, demonstrating practical potential beyond mechanical inoculation.
Limitations of Topical RNAi:
Degradation: DsRNA applied to plant surfaces is vulnerable to rapid degradation from environmental factors such as UV radiation and nucleases, resulting in a limited window of efficacy (typically 5–7 days).
Delivery Challenges: While foliar spray is easy, ensuring efficient uptake of dsRNA into plant cells can be a hurdle. Most successful studies have relied on mechanical co-inoculation with the virus, which is not suitable for practical field application in disease management.
Cost: The current cost of producing dsRNA as the biologically active agent is still a subject of research to ascertain its economic feasibility for widespread use.
Persistence: For sustained protection, repeated applications may be necessary unless novel carriers are developed to enhance stability.
Off-target Effects (Potential): Although generally considered low risk, generating dsRNA sequences requires careful screening to ensure they do not possess sufficient homology to unintended gene targets in beneficial organisms (e.g., pollinating bees and butterflies), which could lead to toxic effects.
Ongoing research is focused on developing novel delivery methods (e.g., using layered double hydroxide particles as carriers to protect dsRNA from degradation) and optimizing dsRNA design to overcome these limitations, positioning topical RNAi as a valuable future addition to integrated disease management toolboxes.
What is the structure and genomic organization of TSWV?
Tomato Spotted Wilt Virus (TSWV) is a spherical to pleomorphic RNA virus, typically 80–120 nanometers in diameter. It possesses a lipid bilayer envelope derived from the host membrane, giving it an irregular appearance.
Structure:
Envelope Glycoproteins: The outer membrane of the virion contains two major glycoproteins, Gn and Gc, which are crucial for vector infection (binding to insect host cells) and virion assembly. Under electron microscopy, these appear as spiky projections.
Internal Structure: Inside the envelope, TSWV contains three ribonucleoprotein (RNP) complexes. Each of the three single-stranded RNA segments is loosely coated by numerous nucleocapsid (N) proteins, forming helical coils.
Nucleocapsid (N) Protein: The N protein wraps around the RNA, forming ring-like trimer units that allow for flexibility. Naked RNA without the N protein is non-infectious.
RNA-dependent RNA Polymerase (RdRp or L protein): The L protein, the viral polymerase, remains associated with the RNP complex.
Non-structural Movement Protein (NSm): This protein helps the virus spread between plant cells by creating tubules that span cell walls, facilitating the movement of the viral RNA-protein complex.
Non-structural Silencing Suppressor (NSs): This protein interferes with the host plant’s RNA interference (RNAi) defense mechanisms.
Localization: TSWV particles are typically found in the host cell cytoplasm, often within the endoplasmic reticulum (ER) lumen or associated with viral inclusion bodies.
Genomic Organization: TSWV has a tripartite (three-segment) single-stranded RNA genome, labeled L, M, and S. Each segment is individually packaged as an RNP and enveloped within the virion.
L Segment (~8.9 kb): This is the largest segment and has negative-sense polarity. It encodes a single large open reading frame (ORF) in the viral-complementary sense for the RNA-dependent RNA polymerase (RdRp or L protein), which is essential for viral replication and transcription.
M Segment (~4.8–5.4 kb): This segment has an ambisense arrangement, meaning it contains two non-overlapping ORFs in opposite polarities separated by an AU-rich intergenic region.
One ORF (viral sense) encodes the NSm (movement protein).
The second ORF (viral-complementary sense) encodes the glycoprotein precursor, which is then processed into Gn and Gc glycoproteins.
S Segment (~2.9 kb): This is the smallest segment and also has an ambisense gene layout.
The viral-complementary strand encodes the nucleocapsid (N) protein.
The viral-sense strand encodes the NSs protein (silencing suppressor).
The ambisense organization of the M and S segments allows for regulated, staggered gene expression, with the intergenic region forming a hairpin structure that aids in mRNA stability and translation. Both 5′ and 3′ ends of each segment are complementary, forming a panhandle secondary structure crucial for replication initiation.
What is the life cycle of TSWV, from acquisition to plant infection?
The life cycle of Tomato Spotted Wilt Virus (TSWV) involves a complex interplay between its plant hosts and its thrips insect vectors, encompassing virus acquisition, replication, and spread within both organisms.
1. Acquisition in Larval Thrips:
The cycle begins when larval-stage thrips (specifically, the first and second instars, which last about 1-3 days) feed on a TSWV-infected plant. Only larvae can acquire the virus; adult thrips are unable to become infected.
2. Thrips Infection and Replication:
Once ingested, the TSWV virions bind to specific receptors in the thrips’ gut via their Gn and Gc glycoproteins.
The viral envelope fuses with the membrane of host cell vesicles, releasing the ribonucleocapsid into the cytoplasm of the insect cell.
Inside the thrips’ cytoplasm (the virus does not enter the nucleus), the viral RNA-dependent RNA polymerase (L protein) begins transcribing the genomic RNA segments into mRNAs.
Host ribosomes then translate these viral mRNAs, producing proteins like the nucleocapsid (N) protein, glycoproteins, and more L protein.
Once sufficient N protein is synthesized, it encapsulates newly made genomes and antigenomes to form new ribonucleoprotein (RNP) complexes. These new RNPs are biologically active and serve as templates for further replication.
New virions are assembled as RNPs bud through internal membranes, acquiring their lipid envelope containing Gn/Gc glycoproteins. These virions then exit the insect cell by budding through the insect’s cell membrane.
3. Adult Vector Transmissibility:
Critically, thrips that acquire the virus as larvae remain viruliferous (capable of transmitting the virus) for their entire adult life, which can be 30-45 days. They do not lose infectivity. However, the virus is not passed vertically from adult thrips to their eggs or offspring.
4. Inoculation of Plant:
Infected adult thrips then move to healthy host plants. As they feed, they puncture the plant cells using a “punch-and-suck” method, releasing saliva that contains virus particles directly into the plant cell cytoplasm through the feeding wounds. Inoculation can occur within minutes of feeding.
5. Plant Replication and Spread:
Once inside a plant cell (through wounds or plasmodesmata), the virus uses its own L protein to transcribe viral mRNAs from its encapsidated genomic RNA.
Host ribosomes translate these mRNAs to produce viral proteins (N, NSm, NSs, glycoproteins).
Replication begins when enough N protein is available to encapsulate new genomes and antigenomes into RNP complexes.
These RNPs then move from cell to cell through plasmodesmata, a process aided by the NSm (movement protein).
After spreading locally, the virus reaches the plant’s vascular tissue (phloem) and spreads long-distance, leading to systemic infection throughout the entire plant within days to weeks.
The newly infected plant tissue becomes a source for future virus acquisition by new generations of thrips larvae, completing the cycle.
The timing of the thrips life cycle (egg to adult in 7.5–13 days) means that the critical larval acquisition stage occurs early, with adult thrips subsequently feeding and transmitting the virus. Plant symptoms typically develop 1–3 weeks after inoculation, depending on factors like plant age and environmental conditions.
What factors influence the infection and spread of TSWV in a given area?
Several interconnected factors influence the infection rates and spread of Tomato Spotted Wilt Virus (TSWV), making its management complex:
Inoculum Presence (Virus Source):
Infected Weed Hosts: The presence of infected weed species (e.g., dandelions, sowthistle, chickweed, morning glory, nightshade, lambsquarter, clover) in and around crop fields is a primary driver of TSWV spread. These weeds act as reservoirs, harboring the virus and thrips, especially early in the season, and allowing the virus to overwinter.
Infected Crops/Transplants: Previously infected crops or vegetatively propagated ornamentals and transplant seedlings can also serve as initial sources, introducing the virus to new areas or subsequent growing seasons.
Vector Density and Species:
Thrips Population Size: High populations of TSWV-vectoring thrips (especially Frankliniella occidentalis, F. fusca, and Thrips tabaci) significantly increase the rate of virus spread to crops.
Thrips Behavior: The tendency of thrips to hide within flower buds and top leaves makes them difficult to reach with contact pesticides. Their small size also allows them to be easily carried by wind.
Virus-Vector Fitness: TSWV can manipulate its host plant (e.g., suppressing terpene synthesis, which repels thrips) to attract more thrips, thereby enhancing its own spread. Additionally, the virus may extend the lifespan of infected thrips or increase their egg-laying capacity, further increasing transmission opportunities.
Environmental Conditions:
Temperature: TSWV and its thrips vectors thrive in warm, dry conditions. Warmer temperatures accelerate thrips reproduction and development (egg to adult in 7.5-13 days), leading to faster population growth and more severe outbreaks.
Rainfall: Conversely, rainfall can sometimes suppress immature thrips populations.
Seasonal Incidence: Thrips species may have different seasonal peaks (e.g., onion thrips in early summer, western flower and tomato thrips in mid to late summer), affecting the timing of peak TSWV risk.
Crop and Landscape Factors:
Planting Date: Planting crops during periods of peak thrips emergence drastically increases infection rates, as young plants are most vulnerable. Delaying planting until after peak thrips migration can reduce disease risk.
Host Availability: Growing susceptible crops (e.g., tomato, pepper) near weed hosts or other susceptible crops with confirmed TSWV infection increases the likelihood of spread.
Cultivar Susceptibility: Crops lacking specific resistance genes (e.g., Sw-5 in tomato, Tsw in pepper) are highly vulnerable, allowing rapid virus replication and spread. Even resistant cultivars can be overcome by resistance-breaking strains or show symptoms on reproductive tissues (flowers/fruit) where the gene is not expressed.
Landscape Pattern: TSWV can spread quicker in patchy fields or near weed-infested non-cropped areas, which serve as bridges for thrips movement.
Management Practices:
Ineffective Thrips Control: If thrips populations are not managed effectively early in the season, the virus can spread extensively even before symptoms become visible. Rapid development of insecticide resistance in thrips due to overuse or lack of rotation further complicates control.
Lack of Sanitation: Failure to remove infected plants and control weeds creates continuous sources of inoculum.
A comprehensive integrated pest management (IPM) approach that considers these interacting factors is essential for minimizing TSWV impact.
How does RNAi gene expression occur in TSWV and how does it relate to resistance?
Tomato Spotted Wilt Virus (TSWV) exhibits a sophisticated gene expression strategy, primarily utilizing an “ambisense” coding mechanism for two of its three RNA segments. This strategy is crucial for its life cycle and interaction with host defenses, particularly concerning RNA interference (RNAi) and plant resistance.
TSWV Gene Expression Overview: TSWV has a tripartite, single-stranded RNA genome (L, M, S segments).
L Segment Expression: The large (L) segment is negative-sense. It directly transcribes a full-length messenger RNA (mRNA) from its viral-complementary (vc) strand. This mRNA encodes the RNA-dependent RNA Polymerase (RdRp or L protein), which is vital for replicating the viral genome and transcribing other viral genes.
M Segment Expression: The medium (M) segment uses an ambisense arrangement. It produces two subgenomic mRNAs:
From the viral-complementary (vc) strand: The glycoprotein precursor (Gn/Gc), which is processed into the envelope glycoproteins crucial for thrips infection and virion assembly.
From the viral-sense (v) strand: The NSm (movement protein), which facilitates cell-to-cell movement of the virus within the plant host.
S Segment Expression: The small (S) segment is also ambisense, yielding two subgenomic mRNAs:
From the viral-complementary (vc) strand: The nucleocapsid (N) protein, which encapsulates the viral RNA to form ribonucleoprotein (RNP) complexes.
From the viral-sense (v) strand: The NSs (silencing suppressor) protein, a key virulence factor that interferes with the host’s RNAi defense.
Relationship to RNAi and Resistance:
Host RNAi Response: In plants, RNAi is a primary defense mechanism against viruses. The plant’s Dicer-like enzymes recognize double-stranded RNA (dsRNA) structures from the replicating virus and cleave them into small interfering RNAs (siRNAs). These siRNAs then guide the RNA-induced silencing complex (RISC) to degrade complementary viral mRNAs, thus suppressing viral gene expression and replication.
Viral Silencing Suppressor (NSs): TSWV’s NSs protein is a potent silencing suppressor. It counteracts the host’s RNAi by:
Sequestering siRNAs, preventing them from being loaded onto RISC.
Potentially inhibiting the processing of longer dsRNA precursors into siRNAs.
The full extent of its function is still debated, but it appears to interfere with the amplification of siRNA trigger molecules.
NSs is also a pathogenicity determinant, allowing the virus to overcome natural host defenses.
Interestingly, NSs also plays a role in attracting thrips to infected plants and is essential for persistent infection in the thrips vector, suggesting it may also suppress RNAi in insects.
Transgenic RNAi Resistance: Humans have leveraged the RNAi mechanism to engineer resistance in plants. By introducing a transgene that expresses a dsRNA homologous to a critical TSWV gene (especially the N gene), plants can produce siRNAs that target and silence that viral gene, leading to resistance.
However, the effectiveness is sequence-specific; not all viral genes are equally effective targets (N gene is generally best).
Viruses can evolve to overcome this resistance through spontaneous mutations in the targeted gene, reducing homology with the siRNAs and leading to “resistance-breaking” strains.
Host Resistance Genes (e.g., Sw-5): Natural resistance genes like Sw-5 in tomato often trigger a hypersensitive response (localized cell death) upon recognition of a viral component. The NSm protein of TSWV is known to trigger the Sw-5 resistance gene response, making it a key factor in host-pathogen interactions. Mutations in the NSs sequence can also lead to resistance-breaking isolates that evade recognition by resistance genes like Tsw in capsicum.
- Kim SG, Lee S-D, Lee W-M, Jeong H-B, Yu N, Lee O-J, Lee H-E. Effective Tomato Spotted Wilt Virus Resistance Assessment Using Non-Destructive Imaging and Machine Learning. Horticulturae. 2025; 11(2):132. https://doi.org/10.3390/horticulturae11020132
- Khan, S. (2024, September 19). Tomato spotted wilt virus symptoms and treatments. Envirevo Agritech. https://envirevoagritech.com/tomato-spotted-wilt-virus-symptoms-treatments/
- Extension. (2022, February 25). Tomato Spotted Wilt Virus (TSWV): a virus you should know and be able to identify! Extension. https://extension.unh.edu/blog/2022/02/tomato-spotted-wilt-virus-tswv-virus-you-should-know-be-able-identify
- Ingwell, L. L., & Egel, D. S. (2023, August 23). Tips for managing Tomato Spotted wilt Virus (TSWV). Vegetable Crops Hotline. https://vegcropshotline.org/article/tips-for-managing-tomato-spotted-wilt-virus-tswv-2/
- Srinivasan, R., Abney, Culbreath, A., Kemerait, R., Tubbs, R., Monfort, W., & Pappu, H. (2017). Three decades of managing Tomato spotted wilt virus in peanut in southeastern United States. Virus Research, 241, 203–212. https://doi.org/10.1016/j.virusres.2017.05.016
- Hall, D. (2023, March 14). Best Ways to Identify, control & Prevent tomato spotted wilt virus on tomato plants. The Scientific Gardener. https://thescientificgardener.com/identify-tomato-spotted-wilt-virus-and-control-tomato-plants
- TSWV Major agricultural challenge. (2025, January 14). Trust Seeds. https://www.trustseeds.com/understanding-tswv-and-its-impact-on-crop-yields/
- Plant and Pest Diagnostic Clinic. (n.d.). Plant and Pest Diagnostic Clinic. https://www.clemson.edu/public/regulatory/plant-problem/
- Management – USDA RAMP Project | Tomato spotted wilt virus. (n.d.). https://tswv.caes.uga.edu/usda-ramp-project/management.html
- EVALUATION OF CULTURAL TACTICS, INSECTICIDES, AND PEANUT GENOTYPES FOR THRIPS AND SPOTTED WILT DISEASE MANAGEMENT IN PEANUT. (2015). [Thesis, The University of Georgia]. https://getd.libs.uga.edu/pdfs/lai_pin-chu_201512_ms.pdf
- Managing thrips and tomato spotted wilt in pepper. (2004). In University of Florida, IFAS, Cooperative Extension Service. https://ipm.ifas.ufl.edu/pdfs/TSWV.pdf
- Farmonaut. (2025, June 18). Best Insecticide for thrips | Tomato thrips control Tips. Farmonaut®. https://farmonaut.com/precision-farming/organic-treatment-and-control-managing-tomato-spotted-wilt-virus-and-thrips-in-diverse-crops/
- Philippe Le Mercier, Chantal Hulo, Patrick Masson (content); Edouard de Castro (software). (n.d.). Tospoviridae ~ ViralZone. https://viralzone.expasy.org/9764
- Batuman O, Turini TA, LeStrange M, Stoddard S, Miyao G, Aegerter BJ, Chen L-F, McRoberts N, Ullman DE, Gilbertson RL. Development of an IPM Strategy for Thrips and Tomato spotted wilt virus in Processing Tomatoes in the Central Valley of California. Pathogens. 2020; 9(8):636. https://doi.org/10.3390/pathogens9080636
- Qi S, Zhang S, Islam MM, El-Sappah AH, Zhang F, Liang Y. Natural Resources Resistance to Tomato Spotted Wilt Virus (TSWV) in Tomato (Solanum lycopersicum). International Journal of Molecular Sciences. 2021; 22(20):10978. https://doi.org/10.3390/ijms222010978
- Riley, D. G., & Pappu, H. R. (2000). Evaluation of Tactics for Management of Thrips-Vectored Tomato spotted wilt virus in Tomato. Plant Disease, 84(8), 847–852. https://doi.org/10.1094/pdis.2000.84.8.847
- Culbreath, A., & Srinivasan, R. (2011). Epidemiology of spotted wilt disease of peanut caused by Tomato spotted wilt virus in the southeastern U.S. Virus Research, 159(2), 101–109. https://doi.org/10.1016/j.virusres.2011.04.014
- Caciagli, P. (2008). Vegetable viruses. In Elsevier eBooks (pp. 282–290). https://doi.org/10.1016/b978-012374410-4.00650-6
- Sheida Shalileh, Pamella Akoth Ogada, Dany Pascal Moualeu, Hans-Michael Poehling, Manipulation of Frankliniella occidentalis (Thysanoptera: Thripidae) by Tomato Spotted Wilt Virus (Tospovirus) Via the Host Plant Nutrients to Enhance Its Transmission and Spread , Environmental Entomology, Volume 45, Issue 5, October 2016, Pages 1235–1242, https://doi.org/10.1093/ee/nvw102
- Alberta Agriculture and Forestry. (2018). Emerging virus and viroid disease risks on greenhouse crops [Report]. https://open.alberta.ca/dataset/20f25b60-cd74-47d6-be5d-e8592f1b0097/resource/7be71cf3-2f32-4704-b7fa-15326c1b69d2/download/200-630-1.pdf
- Gupta, R., Kwon, S., & Kim, S. T. (2018). An insight into the tomato spotted wilt virus (TSWV), tomato and thrips interaction. Plant Biotechnology Reports, 12(3), 157–163. https://doi.org/10.1007/s11816-018-0483-x
- Nilon A, Robinson K, Pappu HR, Mitter N. Current Status and Potential of RNA Interference for the Management of Tomato Spotted Wilt Virus and Thrips Vectors. Pathogens. 2021 Mar 9;10(3):320. doi: 10.3390/pathogens10030320. PMID: 33803131; PMCID: PMC8001667.
- Shahmohammadi N, Khan F, Jin G, Kwon M, Lee D, Kim Y. Tomato Spotted Wilt Virus Suppresses the Antiviral Response of the Insect Vector, Frankliniella occidentalis, by Elevating an Immunosuppressive C18 Oxylipin Level Using Its Virulent Factor, NSs. Cells. 2024 Aug 19;13(16):1377. doi: 10.3390/cells13161377. PMID: 39195265; PMCID: PMC11352781.
- Nachappa P, Challacombe J, Margolies DC, Nechols JR, Whitfield AE, Rotenberg D. Tomato Spotted Wilt Virus Benefits Its Thrips Vector by Modulating Metabolic and Plant Defense Pathways in Tomato. Front Plant Sci. 2020 Dec 18;11:575564. doi: 10.3389/fpls.2020.575564. PMID: 33424878; PMCID: PMC7793759.
- Epidemiology – USDA RAMP Project | Tomato spotted wilt virus. (n.d.). https://tswv.caes.uga.edu/usda-ramp-project/epidemiology.html