Testing of Disinfectants/ Evaluation of disinfectants definition.
- The process of establishing documented evidence that a disinfectant will consistently remove or inactivate known or possible pathogens from samples.
- This complex test is done to determine the effectiveness of disinfectants.
- Robert Koch first described this test in 1881’s article Uber Disinfektion.
- Standing disinfectants can cause a loss of potency. However, the disinfectant may also lose its potency if it is added to organic matter. This must be checked for efficacy.
Different methods for Testing of Disinfectants
There are present different tests which can be used to test the efficiency of disinfectants such as;
- Phenol coefficient test
- Carrier tests
- Capacity test
- In-use test
- Practical tests
- British standard tests for quaternary ammonium compounds
- Disk-Diffusion Method
1. Phenol coefficient test
- This method is sometimes called FDA method or AOAC phenol-cocfficient. These abbreviations are for the Association of Official Agricultural Chemists and Food and Drug Administration.
- This is a good way to test disinfectants that are dissolvable in water. It also allows you to measure their antimicrobial activity using a method similar as that of phenol.
- This procedure uses a particular strain of Salmonella typhi and Staphylococcus aureus as the test organism.
Methods of Phenol coefficient test
There are two distinct methods of Phenol coefficient test, such as;
- Rideal Walker method
- Chick Martin test
A. Rideal Walker method for Phenol coefficient test
- Rider and Walker developed this method of phenol coefficient testing to test the effectiveness of disinfectants against phenol to kill Salmonella Typhi.
- The phenol coefficient can be determined by diluting the disinfectant that sterilizes the suspension of S. Typhi within a certain time and then dividing it by the dilution phenol, which sterilizes both the suspension and the surrounding environment.
Procedure of Rideal Walker method
- After a series (5 ml/tube) of disinfectant dilutions, 0.5 ml 24-hour broth culture of the test organism are added.
- Similar additions in the same amounts are also made to a series dilutions.
- All tubes (disinfectant + and phenol+ organisms) should be placed in a 20°C water bath.
- Subcultures are performed at intervals of 5-10 and 15 minutes.
- Subculture tubes inoculated with the virus are incubated, and then examined for growth.
- The highest dilution (in phenol) of disinfectant that kills the organism in the fastest time is the one with the highest dilution.
- This division gives us the phenol coefficient for the substance being tested.
- After 7.5 minutes, the test bacteria was killed with the disinfectant at a concentration of 1:600. The test organism was also killed with phenol at a concentration of 1:100. This will give the Phenol coefficient of 600/100 = 6. This indicates that the test disinfectant is able to be diluted six-fold as much as phenol, yet still have the same killing power as the organism.
Advantages of the Rideal-Walker test
- This test is highly reproducible and provides a detailed description of the coefficient.
- It is easy to do and it can be done quickly.
- It is possible to quantify the effectiveness of the disinfectant.
Disadvantages of the Rideal-Walker test
- There is no organic matter.
- Salmonella typhi, a microorganism, may not be suitable.
- It takes very little time to disinfect.
- It should only be used for the evaluation of phenolic disinfectants.
- This is truer for phenolic compounds that it is for formaldehyde.
- There are more chances of air contamination being sampled.
- They are not suitable for gram-positive organisms because they are more resistant.
B. Chick Martin test for Phenol coefficient test
- This is a modified Rideal-Walker test in which disinfectant acts in the presence organic contaminants (e.g. dried yeast, feces etc.). To simulate natural conditions.
- The test determines the phenol value of the disinfectant.
- The disinfectants in Rideal Walker’s method are not tested in water. Instead, they act in yeast suspension (or 3% dried human stool) to simulate organic matter.
- Subculture takes 30 minutes. The organism used to test effectiveness is S.typhi.
- The Rideal Walker method gives a lower phenol coefficient.
Procedure
- This yeast suspension is used in the preparation of the test reaction mixture. It is because the activity of disinfectants is significantly reduced or depressed when there is organic matter such as yeast extract.
2. Carrier tests
- It is the oldest test.
- This test was first described by Robert Koch.
Carrier tests Procedure
- These tests are performed on a carrier, such as silk or catgut threads or penicylinders (a small stick), that has been submerged in a liquid culture.
- After drying, the carrier is placed in direct contact with disinfectant for a specified time.
- It is then cultured in a nutrient buffer. No growth indicates disinfectant activity, while growth indicates failure.
- Multiplying the number and contact times of disinfectant tests can give you a potential active concentration-time relationship.
Limitation of the carrier tests
- It is difficult to determine the number of bacteria that has been dried on a carrier.
- It is not possible for bacteria to survive on the carrier after drying.
Carrier tests Example
One example of a carrier test would be the 1990 use-dilution test of American Association of Official Analytical Chemists (AOAC).
The AOAC Use-dilution test
- It is a type carrier-based test.
- The organisms used are Salmonella cholerasuis, S. aureus and P. aeruginosa.
- To confirm the effectiveness of disinfectant dilution, phenol coefficient test results are used.
The AOAC Use-dilution test Procedure
- Carriers are stainless steel cylinders that have been meticulously cleaned and sterilized in an aspargine solution. After cooling, they are inoculated with the test organism by being immersed in one of the culture suspensions.
- The cylinders should be drained on filter papers, dried at 37o C 40 minutes, then exposed to disinfectant for 10 min. Finally, culture the cylinders to determine if the bacteria survived.
- One test is one that compares 60 inoculated carriers (one organism), to one product sample.
- Six carriers are required for the estimation of carrier bacterial load. 6 additional carriers can be added to this list.
- A total of 72 seeded carrier are needed to complete a single test.
- The phenol coefficient test result is confirmed if there is no growth in any of the ten tubes.
- If there is any growth in the carrier, it must be tested again using a lower concentration of the disinfectant.
3. Capacity tests
- The disinfectant is repeatedly tested for its ability to kill.
- Capacity tests simulate practical scenarios of housekeeping or instrument disinfection.
Capacity tests Example
The best-known capacity test is the Kelsey-Sykes test which was discovered by Kelsey and Sykes, in 1969.
Kelsey-Sykes test
- This triple-challenge test is used to determine disinfectant concentrations that are effective in both clean and dirty environments.
- Three successive additions to a bacterial suspension are made to the disinfectant during the course. It takes more than 30 minutes to complete the test.
- The addition of organic matter (autoclaved yeast cell) reduces the concentration by half. This builds up to 0.5%.
- Depending on the disinfectant used, a single organism from each of the following is chosen: S. aureus (P. aeruginosa), P. vulgaris, or P. vulgaris.
- You can perform the procedure under either ‘clean’ and ‘dirty’ circumstances.
- For clean conditions, the disinfectant is diluted in hard water and yeast suspension.
- At 0, 10, and 20 minute intervals, test organism is added either with yeast or alone.
- The contact time between disinfectant and test organism takes 8 minutes.
- The capacity test by Sykes and Kelsey provides a guideline for the dilution.
- This test has a disadvantage: It is quite complicated.
Kelsey-Sykes test Procedure
- Each challenge has three sets of five replica cultures. They are incubated at 32oC 48 hours. Growth is measured by turbidity.
- The disinfectant is evaluated on the ability to kill microorganisms.
- Negative results are those that have more than one negative culture in a set. If negative results are found after the second and third challenges, the disinfectant passes the dilution test.
- Although the third challenge is not part of the pass/fail criteria, positive cultures are considered to be inbuilt controls.
- A lower concentration of disinfectant might be used if positive cultures are not found after the third challenge.

4. Practical tests
- After measuring the time-concentration relationship for the disinfectant in a quantitative suspension, practical tests are conducted under real-life conditions.
- It is important to determine if the proposed use of dilution is still appropriate under the conditions in which it will be used.
Example of Practical tests
The best known practical tests are the surface disinfection tests.
Surface disinfection tests
- Surface tests are used to determine the effectiveness of the sanitizer against surface-adhered microorganisms.
- Surface tests can be used to determine in-use conditions such as contact times, temperatures and use-dilutions.
Surface disinfection tests Procedure
- Th
- A small tile, a microscopically-sized slide, or a piece PVC with a stainless steel disc as the test surface. The test surface is infected with a standard inoculum of the bacteria and dried.
- After that, a volume of disinfectant solution must be distributed on the carrier.
- The number of survivors after the exposure time is determined using an impression on a contact sheet or a rinse technique in which the carrier is rinsed with a diluent and the bacteria count is determined in the rinsing liquid.
- A control series in which the disinfectant has been replaced by distilled water is included to measure the rate of spontaneous death of organisms dried on carriers.
- The quantitative reduction can be determined by comparing the survivors of this control series to the test series.
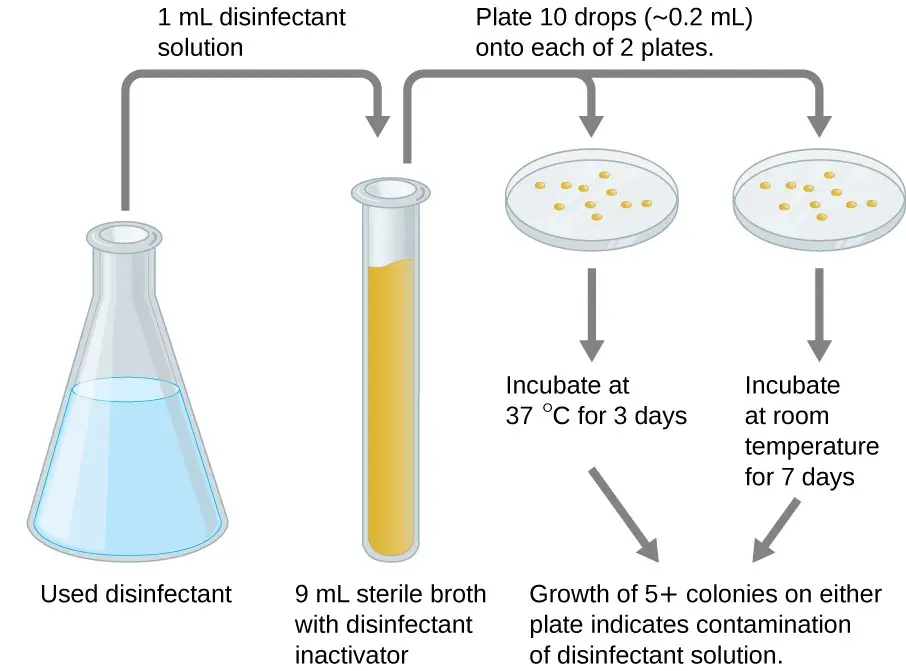
5. In-use test / Kelsey–Maurer test
- Maurer, in 1985, described a simple test that can be used in hospitals or laboratories to detect the presence of disinfectants.
- The Kelsey-Maurer “in-use” test (Kelsey-Maurer test) is a test that determines if the disinfectant chosen is effective in actual hospital practice as well as for the duration of its use.
- The disinfectant’s ability to kill a predetermined number of pathogenic staphylococci at a surface in a specified time period determines its effectiveness.
- The in-use test is more reliable than the phenol coefficient test and allows for a more precise determination of disinfectant effectiveness.
In-use test / Kelsey–Maurer test Procedure
- To the 9 ml of diluent, a 1 ml amount of disinfectant is added.
- Each of the two nutrient-agar plates should have ten drops of each 0.02 ml volume of the diluted mixture.
- The one at 37oC is incubated for three days, the other at room temp for seven days.
- Contamination is indicated by five colonies or more on each plate
6. British standard tests for quaternary ammonium compounds
- This test was initially described in 1960 to distinguish bactericidal action from the high level of bacteriostatic activity, whi
- The 1960 discovery of this test allowed for the distinction between bactericidal and bacteriostatic action, which is characteristic in QACs.
- The test can also be applied to synthetic phenols and chlorhexidine.
- Inactivator contains 2% lecithin, 3% non-ionic detergent (polysorbate80).
- The suspensions of gram-negative and gram-positive bacteria are used for the test.
- A series of samples taken from disinfectant containing 5×108 to 5×109 bacteria per milliliter at the beginning of the test can be used to create a death curve. This is based on colony counts and reduction factors of up to 106 (99.99% kill).
- To determine the antimicrobial properties of QAC, the lowest dilution of disinfectant was chosen that will, in test conditions, reduce the microbial population by not more than 0.01% compared to the control.
- This revision used E. coli and took 10 minutes to contact.
- E.coli culture suspension with equal amounts of horse serum is the challenge medium.
- One ml is added to nine milliliters of each dilution. Two control tubes contain the disinfectant alone.
- After the exposure period ends, add one ml of each mixture to 9 ml inactivator. The surviving bacteria are then counted on agar plates as colony-forming units.
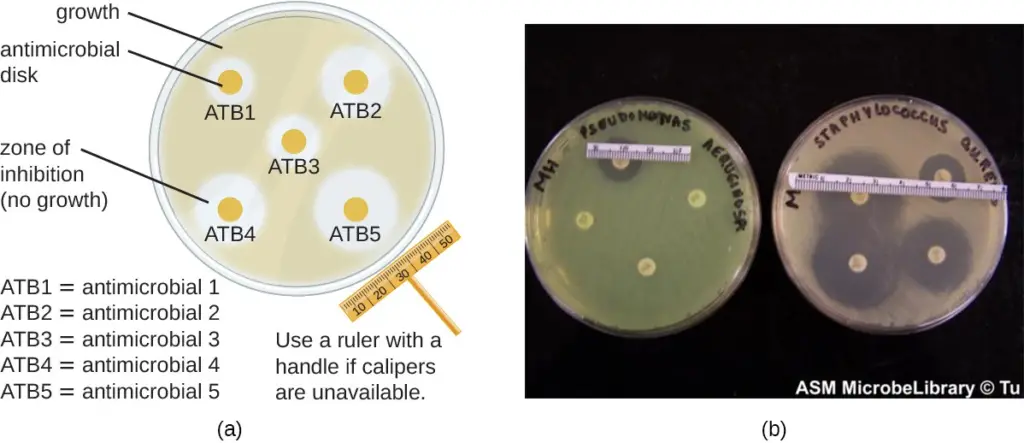
7. Disk-Diffusion Method
- This involves using various chemicals to separate sterile filter paper disks.
- Next, place the disks on an inoculated agar plate. The chemicals are released from the disks onto the agar.
- Clear areas around the disks are zones of inhibition as the bacteria “lawn” grows.
- There are many factors that influence the size of the inhibition zones, such as whether the agent can dissolve in water or diffuse well in the agar. However, larger inhibition zones usually correlate with greater inhibition effectiveness.
- Each zone’s diameter is measured in millimeters.
References
- https://www.slideshare.net/kmv/testing-of-disinfectants
- https://www.koshic.org/event/file/2013_17/3_02.pdf
- https://www.slideshare.net/IkennaGodwin/evaluation-of-disinfectant-76555655
- https://courses.lumenlearning.com/microbiology/chapter/testing-the-effectiveness-of-antiseptics-and-disinfectants/
- https://www.microrao.com/micronotes/pg/testing_of_disinfectants.pdf
- https://www.cambridge.org/core/services/aop-cambridge-core/content/view/6417853D22D6AA767279B733EE45E9D3/S0950268805004231a.pdf/div-class-title-3-the-chick-martin-test-for-disinfectants-chick-h-martin-c-j-hyg-1908-span-class-bold-8-span-654-697-div.pdf
- The testing of disinfectants: Gerald Reybrouck, International Biodeterioration & Biodegradation 41 (1998) 269-272
- Joan F.Gardner, Margaret M Peel. 1991. Introduction to sterilization and disinfection control, 2nd edition, Churchill Livingstone
- https://slidetodoc.com/disinfectant-silpa-m-assistant-professor-dept-of-pharmacognosy/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.