Sulphur cycle Definition
- The sulphur cycle is a biogeochemical cycle that involves the movement of sulphur between rocks, waterways, and living organisms.
- Sulphur is one of the earth’s most prevalent elements. This non-metal is yellow, brittle, odourless, and tasteless. Sulfur is present in all protein types.
- Methionine, cystine, and cysteine are sulphur-containing amino acids that are directly absorbed by plants.
- The release of sulphur into the atmosphere is caused by the combustion of fossil fuels, volcanic activity, and the degradation of organic molecules.
- Sulfur is stored in subsurface rocks and minerals on land. It is released as a result of precipitation, rock weathering, and geothermal vents.
- Sulfur compounds can be used as oxidants or reductants in microbial respiration.
- The global sulphur cycle involves the change of sulphur species via several oxidation states, which play a significant role in geological and biological processes.
- The sulphur cycle is described as follows:
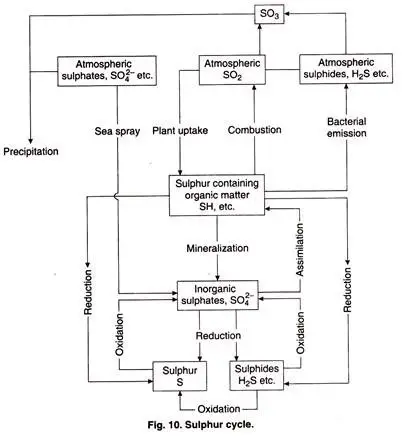
- By the weathering of rocks, sulphur is liberated.
- When sulphur comes into contact with air, sulphates are formed.
- Plants and microorganisms absorb sulphates and transform them into organic compounds.
- The organic form of sulphur is subsequently ingested by animals via their food, so transferring sulphur up the food chain.
- When the animals die, part of the sulphur is released by decomposition and some enter the tissues of microorganisms.
- There are various natural sources such as volcanic eruptions, evaporation of water, and breakdown of organic waste in wetlands, that release sulphur straight into the atmosphere. This sulphur falls to earth with rainfall.
Sulfur Cycle Overview
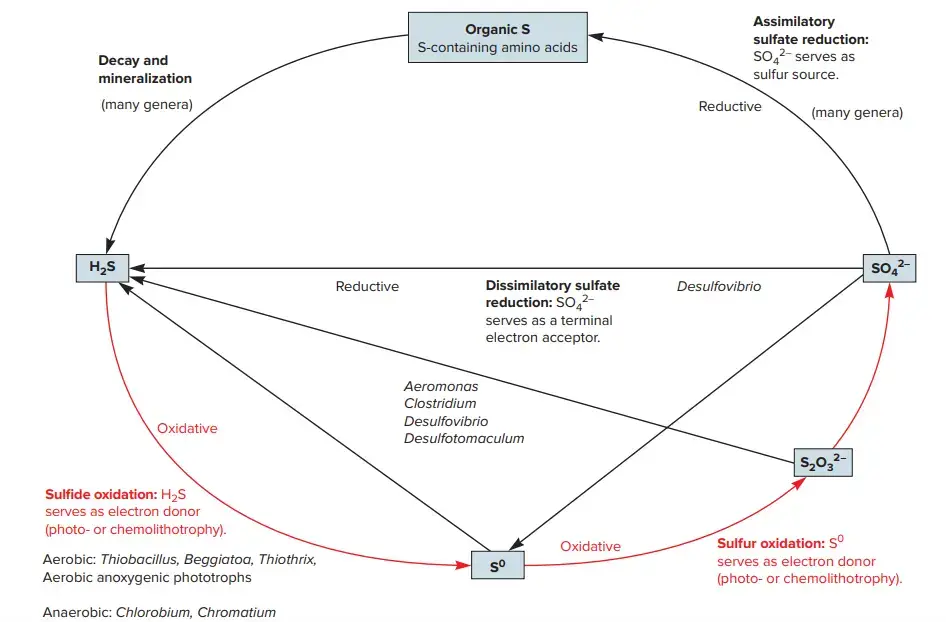
- Microorganisms play a significant role in the sulphur cycle; a simplified form is depicted in the picture.
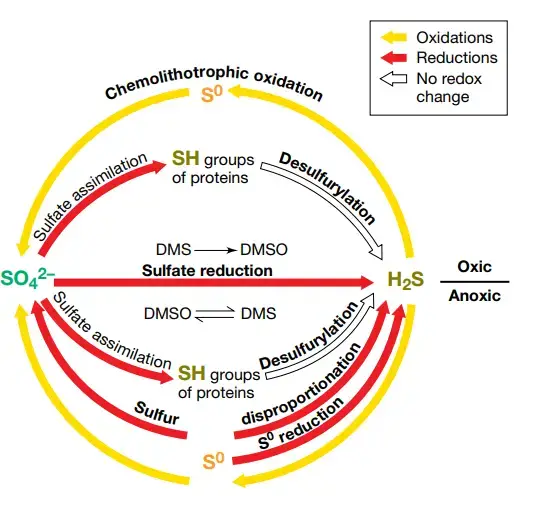
- Similar to the nitrogen cycle, sulphur can act as an electron acceptor, electron donor, or both, depending on its oxidation state.
- Assimilatory sulphate reduction refers to the process by which plants and microorganisms reduce sulphate, the completely oxidised species, for use in amino acid and protein production.
- When sulphate diffuses into anoxic settings, microbial dissimilatory sulphate reduction becomes possible.
- During anaerobic respiration, sulphate serves as a terminal electron acceptor for a number of microorganisms, including -proteobacteria such as Desulfovibrio and Desulfonema spp. and archaea belonging to the genus Archaeoglobus.
- This leads in the buildup of sulphide. Fully reduced sulphide can subsequently serve as an electron supply for anoxygenic photosynthetic microorganisms and chemolithoautotrophs, such as members of the Chlorobi phylum and the Thiobacillus genus, respectively.
- These microorganisms convert sulphide to sulphur and sulphate. Other bacteria have been discovered to reduce elemental sulphur (S0) via dissimilatory means.
- In hypersaline sediments, these organisms include Desulfuromonas, thermophilic archaea, and cyanobacteria. Sulfite (SO32) is an additional essential intermediate that can be converted to sulphide by a wide range of microbes, including representatives of the genera Alteromonas, Clostridium, Desulfovibrio, and Desulfotomaculum.
- Dimethylsulfoniopropionate (DMSP) is a crucial sulfur-containing chemical molecule. It is produced as a compatible solute by marine phytoplankton. Bacterioplankton (floating bacteria) use DMSP as a carbon and sulphur source when these cells die and release it.
- It is converted to dimethylsulfide (DMS) and then discharged into the atmosphere.
- There, DMS is swiftly transformed into a number of sulphur compounds that serve as nuclei for water droplet production and contribute to cloud formation.
- It is theorised that enhanced DMS production could offset the consequences of global climate change because clouds help keep the Earth’s surface cool.
Types of Sulphur Cycle
There are two major kinds of the Sulphur Cycle, namely;
1 . Gaseous Sulphur Cycle
- Through bacterial emission (H2S), fossil fuel combustion (SO2), wind-blown sea salts (SO 2-4), and volcanic emissions, sulphur reaches the atmosphere (H2S, SO2, SO2-4).
- The majority of sulphur existing as SO2 or H2S is transformed to SO3, which dissolves in water droplets to generate sulphuric acid.
- Due to the use of fossil fuels, the sulphur cycle is overburdened.
- As a result, SO2 discharged into the atmosphere forms a sizeable portion of total sulphur transport on a global scale. This higher level of sulphur is converted to sulphuric acid in rainwater, resulting in negative ecological repercussions.
2. Sedimentary Sulphur Cycle
- In the sedimentary phase, the weathering and degradation of inorganic and organic deposits liberates sulphur.
- The SO2-4 ion transports sulphur to terrestrial and aquatic habitats. After being absorbed from the soil by plants and microbes, sulphate ion is reduced and ultimately integrated as the sulphydryl group (-SH) in proteins. SO
- Desulphovibrio bacteria on the ocean floor convert certain sulphates straight to sulphides, H2S, or elemental S under anaerobic circumstances.
- This H2S escapes into the atmosphere and refills the sulphur lost by precipitation.
- Bacteria of the genus Thiobacillus oxidise H2S with O2 to produce sulphates. Sulfur in excess mixes with water to produce acidic rain.
Sulphur Cycle Steps
The key phases of the sulphur cycle are as follows:
1. Decomposition of Organic Compounds
- Protein breakdown generates sulfur-containing amino acids.
- Desulfotomaculum bacteria convert sulphates into hydrogen sulphide (H2S).
2. Oxidation of Hydrogen Sulphide to Elemental Sulphur
- Hydrogen sulphide oxidises to yield elemental sulphur.
- The oxidation process is initiated by certain photosynthetic bacteria of the families Chlorobiaceae and Chromatiaceae.
3. Oxidation of Elemental Sulphur
- The elemental sulphur found in the soil cannot be directly utilised by plants.
- Consequently, chemolithotrophic bacteria transform it into sulphates.
4. Reduction of Sulphates
- Desulfovibrio desulfuricans converts sulphates into hydrogen sulphide.
- This takes place in two steps:
- Initially, ATP is used to convert sulphates to sulphites.
- The conversion of sulphite to hydrogen sulphide is the second step.
Economic importance of sulphur:
- Each year, millions of tonnes of sulphur are manufactured, primarily for the production of sulfuric acid, which has widespread industrial applications.
- Because of its propensity to operate as an oxidising or reducing agent, sulphur is directly involved in the formation of fossil fuels and the majority of metal deposits.
- One of the limiting considerations for the concentration of precious metals in a source is the presence or absence of sulphur.
Impact of Human Activities on Sulphur cycle
- The global sulphur cycle is greatly influenced by human activity.
- The global sulphur cycle is greatly influenced by human activity.
- Due to the combustion of coal, natural gas, and fossil fuels, the amount of sulphur in the atmosphere and ocean has greatly increased.
- In addition, the sedimentary rock sink has been depleted.
- Without the influence of humans, sulphur would stay held in rocks for millions of years until it was uplifted by tectonic activity and then released by weathering and erosion.
- However, it is currently being excavated and burned at an alarming rate.
- Sulphate deposition has increased by a factor of 30 in the most polluted regions of the planet.
- Since the beginning of the 20th century, coal and petroleum have been extracted at such a rapid rate that the world sulphur flow has doubled.
References
- Jørgensen, B. B., Findlay, A. J., & Pellerin, A. (2019). The Biogeochemical Sulfur Cycle of Marine Sediments. Frontiers in Microbiology, 10. doi:10.3389/fmicb.2019.00849
- Loka Bharathi, P. A. (2008). Sulfur Cycle. Encyclopedia of Ecology, 192–199. doi:10.1016/b978-0-444-63768-0.00761-7
- Brimblecombe, P. (2015). BIOGEOCHEMICAL CYCLES | Sulfur Cycle. Encyclopedia of Atmospheric Sciences, 187–193. doi:10.1016/b978-0-12-382225-3.00015-3
- https://www.vedantu.com/geography/sulfur-cycle
- https://prepp.in/news/e-492-sulphur-cycle-environment-notes
- https://www.biologydiscussion.com/environment/sulphur-cycle/sulphur-cycle-types-2-major-types-of-sulphur-cycle-environment/16639
- https://www.cliffsnotes.com/study-guides/biology/plant-biology/biogeochemical-cycles/the-sulfur-cycle
- https://unacademy.com/content/upsc/study-material/general-awareness/sulphur-cycle-definition-steps-diagram-importance-and-facts/
- https://www.space4water.org/s4w/web/water/sulphur-cycle
- https://courses.lumenlearning.com/wm-biology2/chapter/the-sulfur-cycle/
- https://www.britannica.com/science/sulfur-cycle
- https://en.wikipedia.org/wiki/Sulfur_cycle
- lenntech.com/sulphur-cycle.htm
- https://www.clearias.com/sulphur-cycle/