Aspergillus is a genus and consists of 837 species of fungi. They can be found in different climates worldwide. In 1729 by the Italian priest and biologist Pier Antonio Micheli first listed Aspergillus. They are the asexual spore-forming structure that is common to all Aspergillus species.
Lactophenol Cotton Blue (LPCB) is a widely employed mounting technique for the microscopic study of fungi. The preparation of LPCB is a simple process.
Study of Aspergillus by Using Temporary Mounts
Aim
To prepare a temporary mount of aspergillus in order to examine the shape, structure, and motility character of Aspergillus.
Principle
The Lactophenol Cotton Blue solution stains the chitinous cell wall of the fungal cell, which makes them different from the surrounding environments and can easily distinguishable. LCB is made of three main components such as Phenol, Lactic acid, and Cotton blue.
In LCB, the phenol functions as a disinfectant by inhibiting other living organisms. Lactic acid helps to preserve the fungal structures and the Cotton blue stains the chitin, which is the main component of the fungal cell wall.
Requirement
For this practical Lactophenol Cotton Blue solution is required which can be prepared by following these steps.
Procedure
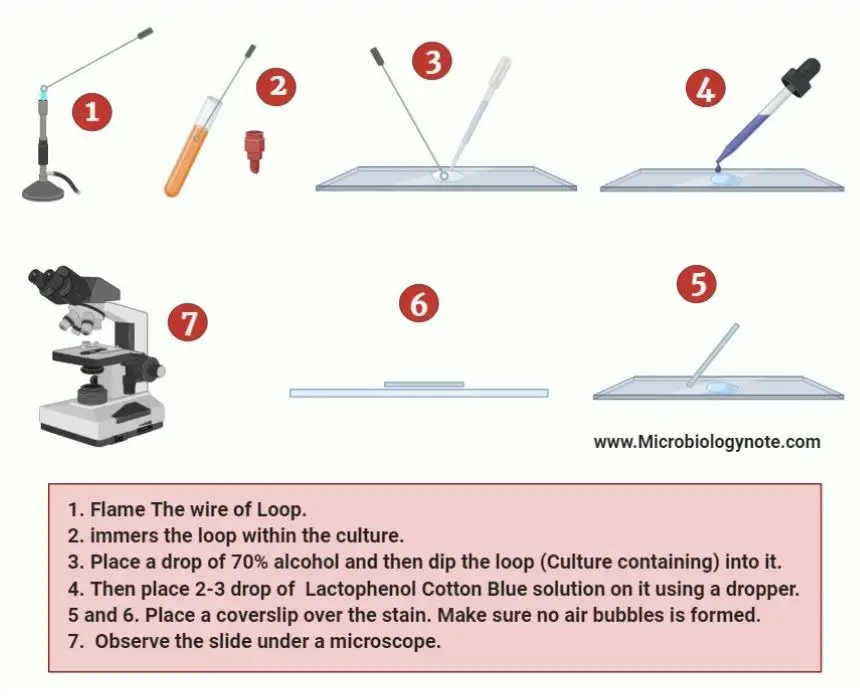
- Take a clean microscope slide and place a single drop of 70% alcohol on it.
- Now transfer the Aspergillus from its parent culture to the drop of alcohol by using a sterile inoculation loop.
- Before transfer, hold the wire portion of the inoculation loop on the flame until it glows red hot. Then wait 10 sec until the wire is cooled and it is ready to dip into the parent culture of aspergillus.
- After that, remove the cap or cotton plug from the culture tube and flame the neck of the tube to make sure all contaminating microorganisms are killed. Then dip the loop inside the tube.
- Before Replace the cap, flame the neck of the tube to kill the other microorganisms.
- Now immerse the loop containing fungal culture into the drop of 70% alcohol.
- To make sure the sample is properly mixed with the alcohol, tease them by using a needle mounter.
- Then place one or two drops of Lactophenol Cotton Blue Solution on the mixture of fungal cell and alcohol with the help of a sterile dropper or pipette.
- Carefully place a clean coverslip over the stain and make sure no air bubbles are formed.
- Now use the low-power objective lens in the microscope to perform the initial examination.
- After that use 40X objective for a more precise study of spores and other structures.
Observation
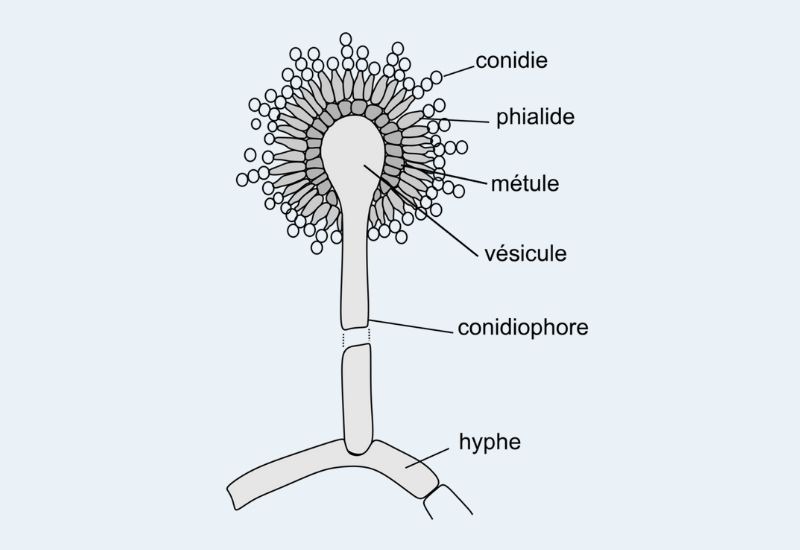
Different parts of staining fungus cell are observed under the microscope such as conidia, hypae, vesicule as well as conidiophore, etc.
Result
Based on the observation the stained fungal cell is Aspergillus sp.

Precautions
- Flame the neck of the tube after removal of the cap and before replacing the cap.
- Don’t put the tube cap over the table.
- Perform the test within a laminar airflow cabinet.
- Before immersing the loop into the culture, flame the wire of loop until it gets red hot.
- After transfer of inoculation (Culture) again flame the loop wire.
- Carefully place the coverslip to prevent the formation of air bubbles.
References
- https://microbiologynote.com/lactophenol-cotton-blue-staining-principle-procedure-result/
- https://senthilprabhu.blogspot.com/2017/03/core-practical-fungal-satining.html
- https://laboratoryinfo.com/lactophenol-cotton-blue-lpcb/
- https://en.wikipedia.org/wiki/Aspergillus
- https://mycology.adelaide.edu.au/laboratory/lacto/
- https://www.microscopemaster.com/aspergillus.html