What is Seliwanoff’s Test?
Seliwanoff’s Test is a fundamental biochemical assay designed to distinguish between aldose and ketose sugars based on their structural differences. Named after chemist Theodor Seliwanoff, this test leverages the principle that ketoses (sugars with a ketone group, like fructose) dehydrate more rapidly than aldoses (sugars with an aldehyde group, like glucose) when heated with a reagent containing resorcinol and concentrated hydrochloric acid (HCl). The reaction produces a cherry-red color for ketoses within minutes, while aldoses may show a faint pink hue only after prolonged heating, making timing critical to avoid false positives.
The Seliwanoff test procedure involves mixing the sugar sample with the reagent and heating it briefly in a water bath. A positive result (rapid red coloration) confirms the presence of ketoses such as fructose or sucrose, which hydrolyzes into fructose and glucose under acidic conditions. In contrast, glucose (an aldose) typically yields a negative result, though extended heating may cause isomerization to fructose, leading to misleading outcomes.
This test is widely used in biochemistry and food science to identify sugars like fructose in fermentation media or verify sweeteners in products. For example, sucrose (a disaccharide of glucose and fructose) tests positive due to its fructose component, while maltose (composed of two glucose units) remains negative. However, the test cannot differentiate between specific ketoses, and starch (a polysaccharide) may require prior hydrolysis to yield detectable sugars.
By focusing on reaction speed and color intensity, Seliwanoff’s Test offers a simple yet reliable method for carbohydrate analysis, emphasizing precision in both reagent preparation (0.05% resorcinol in 3N HCl) and controlled heating times to ensure accuracy. Its applications span educational labs, quality control, and research, underscoring its enduring relevance in biochemical studies.
Objectives of Seliwanoff’s test
- To identify ketohexoses in a sample.
- To identify ketones from aldoses.
Principle of Seliwanoff’s test
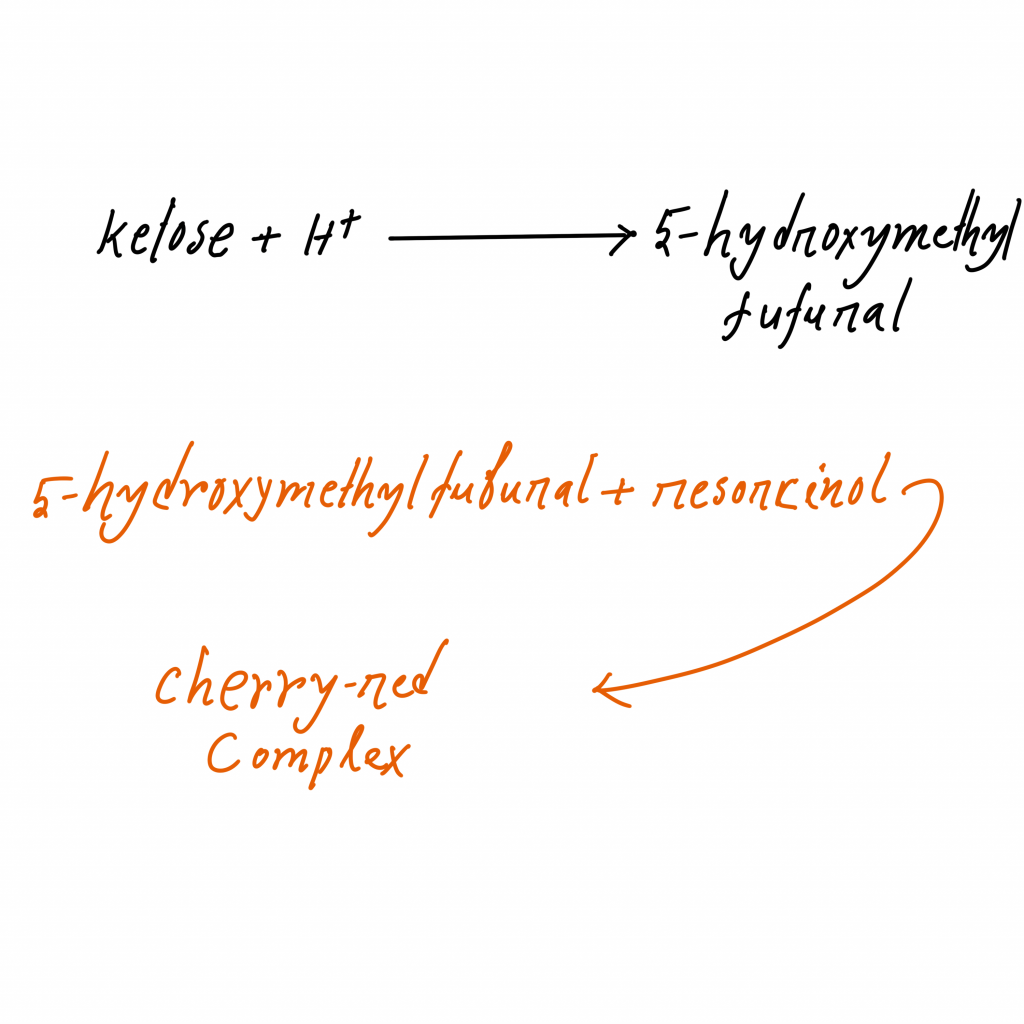
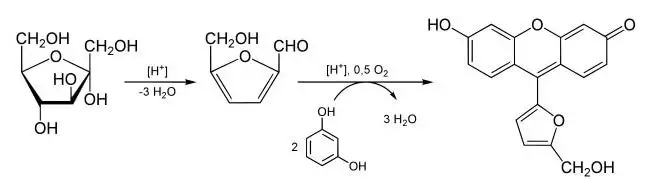
Seliwanoff’s Test uses structural variations between aldoses and ketoses—two varieties of sugars categorised according to the location of their carbonyl group. The test depends on how these sugars behave under extreme heat and acidity. Here the chemical detective is the reagent, a combination of resorcinol and concentrated hydrochloric acid (HCl). Heated, the HCl breaks down and dehydrates the sugar. Because their ketone group is more readily dehydrated into a molecule known as hydroxymethylfurfural (HMF), ketoses—including fructose—react more quickly. This HMF then hooks itself to resorcinol, producing a striking cherry-red hue in minutes. Conversely, aldoses—like glucose—first undergo a longer isomerisation to generate a ketose structure before dehydration, which delays or weakens the reaction—often light pink.
The secret is timing: aldoses lag whereas ketoses “light up” rapidly from their direct response route. Prolonged heating can, however, muddle this difference as aldoses finally generate enough HMF to resemble a ketose outcome. This makes regulated reaction time really vital. Relying on the speed and intensity of the colour shift to categorise sugars in food or biological materials, the test is very helpful. Basically, it’s all about how quickly the sugar’s structure collapses under acid and heat—and if that unravelling generates a strong red flag for ketoses.
Requirements for Seliwanoff’s test
Reagents Required:
- Seliwanoff’s reagent: Dissolve 50 mg of resorcinol (m-hydroxybenzene) in 33 mL of concentrated hydrochloric acid (HCl), then dilute the solution to a final volume of 100 mL with distilled water. In 3N HCl, this produces a 0.05% resorcinol solution.
Materials Required:
- Clean, dry test tubes
- Test tube stand
- Pipettes for accurate measurement
- Distilled water for reagent preparation and control samples
- Test solutions containing carbohydrates such as glucose, fructose, or sucrose
Equipment Required:
- Water bath capable of maintaining a consistent boiling temperature
- Timer to monitor reaction time
Procedure of Seliwanoff’s test
- First, set aside two dry, clean test tubes. Assign the test sample label one and the blank control label second.
- To the test tube marked “test sample,” add 1 mL of the test solution in second step. Carbohydrates like glucose, fructose, or sucrose could abound in the test fluid.
- Add one millilititer of distilled water to the test tube marked “blank control.” This provides a baseline for contrasting the test sample’s colour change.
- Add 2 mL of Seliwanoff’s reagent to each test tube in turn. Seliwanoff uses 3 N hydrochloric acid with 0.05% resorcinol as his reagent.
- In step five, spend one minute in a hot water bath between two test tubes. Make sure the test tubes are firmly gripped and do not come into direct touch with the heating element.
- After one minute, take the test tubes from the water bath and let them cool somewhat. Use carefully to prevent burns. ‘
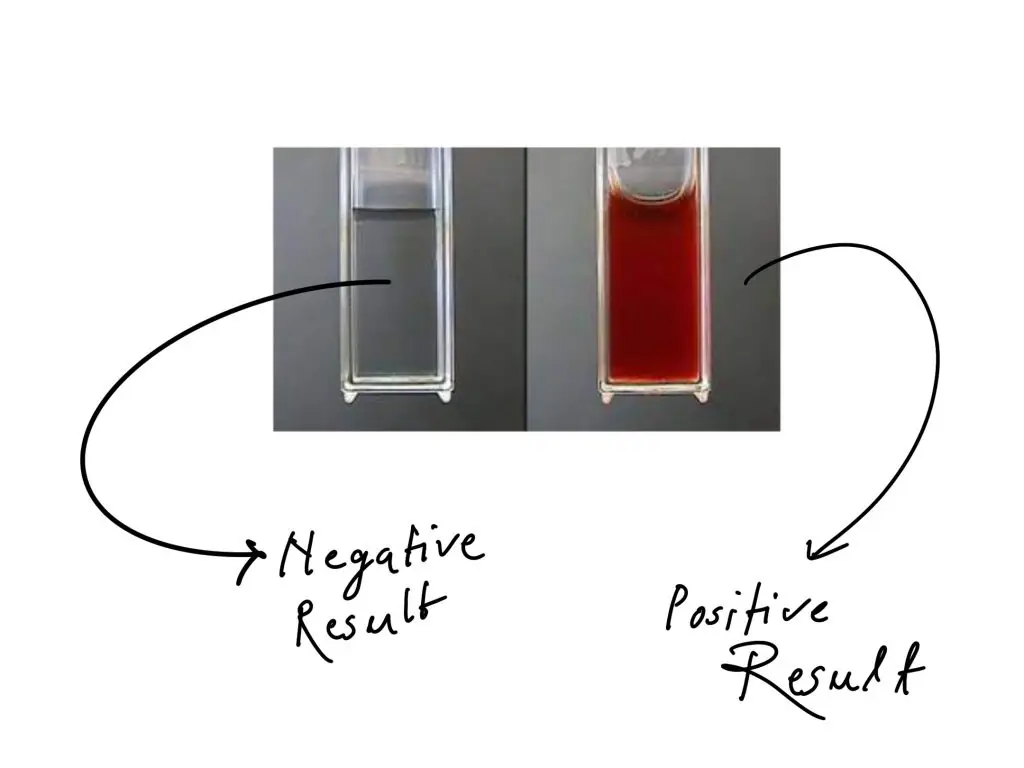
- In the seventh step, note how both of the test tubes change colour. A cherry-red colour points to a favourable outcome, implying the presence of ketoses such fructose or sucrose. A negative result—that is, a light pink hue or no colour change—suggests the existence of aldoses such glucose.
Results of Seliwanoff’s test
- Positive Result: When ketose sugars like fructose or sucrose are present, a strong cherry-red hue quickly forms. The rapid dehydration of ketoses in an acidic environment results in 5-hydroxymethylfurfural, which then combines with resorcinol to generate the red complex, causing this color shift.
- Negative Result: A delayed color shift or weak pink tint indicates aldose carbohydrates like glucose. Under test circumstances, aldoses dehydrate slowly, delaying colored complex formation.
- Interpretation Considerations:
- Aldoses may isomerize into ketoses with prolonged heating, causing false-positive readings.
- If the process takes longer than expected, high aldose concentrations may become bright pink.
| Sugar Type | Expected Color Change | Interpretation |
| Ketose | Rapid cherry-red | Positive |
| Aldose | Slow or no color change; faint pink | Negative |
This test is particularly useful in distinguishing between ketose and aldose sugars in carbohydrate analysis.
Uses of Seliwanoff’s test
- Detects ketose sugars in samples.
- Uses chemical reactivity to distinguish ketose and aldose carbohydrates.
- Used to colorimetrically measure fermentation medium fructose.
- Biochemical labs use it to identify and analyze carbohydrates.
- Used in teaching to illustrate carbohydrate chemistry.
Limitations of Seliwanoff’s test
- High amounts of glucose or other aldoses may interfere with the test by generating similar colored molecules, therefore creating possible false-positive findings.
- Extended boiling may cause glucose to convert to fructose, producing a cherry-red complex and false-positive results.
- The test does not differentiate among various ketose sugars; further tests are needed for particular ketose identification.
- Other carbs such as sucrose and inulin might show benefits as they are broken down by acid to generate fructose, which can cause misunderstanding.
- The test is qualitative and offers no quantitative indication of the ketose sugar content in the sample.

Precautions of Seliwanoff’s test
- To guarantee correct results and avoid contamination, use dry, clean glassware.
- Work in a well-ventilated location or fume hood, handle concentrated hydrochloric acid carefully, don suitable personal protective equipment (PPE), like gloves and safety goggles.
- To keep Seliwanoff’s reagent effective, make new preparations just before usage.
- Steer clear of prolonged heating of the reaction mixture as false-positive results might arise from aldose isomerization to ketoses.
- To guarantee homogeneity of findings, keep constant heating times across all samples.
- Use suitable sugar solution concentrations; too high concentrations might interfere with the test by generating similar colored molecules, hence generating possible false-positive findings.
- To serve as a reference for color change, provide a blank control comprising distilled water and Seliwanoff’s reagent.
- To reduce environmental effect, dispose of chemical waste in line with local laws and institutional safety guidelines.
- https://www.uoanbar.edu.iq/eStoreImages/Bank/2063.pdf
- https://en.wikipedia.org/wiki/Seliwanoff%27s_test
- http://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/carbo/seli/seli.htm
- https://noteshippo.com/seliwanoffs-test-protocol-principle-procedure-result-and-applications/
- https://www.onlinebiologynotes.com/tests-for-specific-carbohydrates-seliwanoffs-test-bials-test-and-iodine-test/
- https://laney.edu/cheli-fossum/wp-content/uploads/sites/210/2012/01/11-Carbohydrates.pdf
- http://www.chem.boun.edu.tr/wp-content/uploads/2014/04/Chem-415-Experiment-1.pdf