Respiratory syncytial virus
- Respiratory syncytial virus (RSV), also known as human respiratory syncytial virus (hRSV) and human orthopneumovirus, is a widespread, contagious virus that causes respiratory tract infections.
- It is a single-stranded, negative-sense RNA virus. It derives its name from the large cells referred to as syncytia that form when infected cells fuse.
- RSV is the leading cause of respiratory hospitalization in infants, and reinfection is prevalent throughout life; it is a notable pathogen for all age groups.
- During the cold winter months, infection rates are typically higher, causing bronchiolitis in infants, the common cold in adults, and more severe respiratory ailments such as pneumonia in the elderly and immunocompromised.
- RSV is capable of causing outbreaks in both community and hospital contexts. After initial infection through the eyes or nostrils, the virus infects the upper and lower airway epithelial cells, causing inflammation, cell damage, and airway obstruction.
- Antigen testing, molecular analysis, and viral culture are among the methods available for the detection and diagnosis of RSV virus.
- Handwashing and avoiding close contact with infected individuals are the primary preventative measures; prophylactic use of palivizumab is also available to prevent RSV infection in neonates at high risk. Currently, there is no vaccine against RSV, despite the fact that several are in development.
- As needed, oxygen therapy and more advanced respiratory support with CPAP or nasal high flow oxygen are used to assist patients with severe illnesses. In severe cases of respiratory failure, intubation and mechanical ventilation may be necessary. Ribavirin is the only licensed antiviral medication for the treatment of RSV in children, though its use remains controversial.
- In 1956, when researchers isolated a virus from chimpanzees with respiratory distress, RSV was first identified. They designated the pathogen as CCA (Chimpanzee Coryza Agent). Robert M. Chanock detected the same virus in children with respiratory illness in 1957.
- Human antibody studies on neonates and children revealed that the infection was prevalent in early childhood. The virus was later renamed human respiratory syncytial virus or human orthopneumovirus (hRSV).
- There are several other pneumoviruses that are extremely similar to hRSV. The most notable is bovine RSV (bRSV), which shares about 80% of its genome with human RSV.
- It shares hRSV’s preference for young animals, causing more severe disease in calves younger than six months. Because bRSV-infected calves present similarly to hRSV-infected children, this has demonstrated to be a valuable animal model for RSV research.
RSV is a member of the family Paramyxoviridae and possesses a continuous, single-stranded, negative-sense RNA genome. Paramyxoviruses are globally significant to both human and animal populations. This family contains several highly contagious viruses, including the human pathogens measles, mumps, and RSV, and the zoonotic viruses Hendra and Nipah. Human RSV (hRSV) is the leading cause of bronchiolitis and pneumonia in infants younger than 12 months. Based on their sequence homology, protein activity, and morphology, RSV and human metapneumovirus (HMPV) belong to the subfamily Pneumovirinae of the Paramyxoviridae family.
Structure of Respiratory Syncytial Virus (RSV)
- Three integral membrane proteins are present in RSV: the receptor attachment glycoprotein (G), the fusion protein (F), and a short hydrophobic (SH) protein. The G protein is responsible for viral attachment to the host cell, whereas the F protein is in charge of fusion. SH is an ion channel composed of five subunits.
- Similar to other paramyxoviruses, it is believed that the polymerization of the matrix protein (M) promotes RSV assembly and budding. M is a membrane-associated protein with positively charged and hydrophobic domains essential for cytoplasmic membrane binding.
- M is essential for the formation of virus particles. Recent research on an M-null mutant has revealed that the absence of M hinders the formation of lengthy viral filaments. This may indicate that the presence of M or the polymerization of M at budding sites encourages the elongation of filamentous viruses.
- M also interacts with the amino-terminal domain of G and the cytoplasmic tail of the F protein, coordinating their recruitment to assembly and budding locations. For some paramyxoviruses, such as PIV1, Sendai, and RSV, matrix proteins are sufficient for the emergence of viruslike particles (VLPs) in tissue culture.
- For optimal budding process efficiency, other paramyxoviruses, such as measles and PIV5, require an accessory protein, such as F or N.
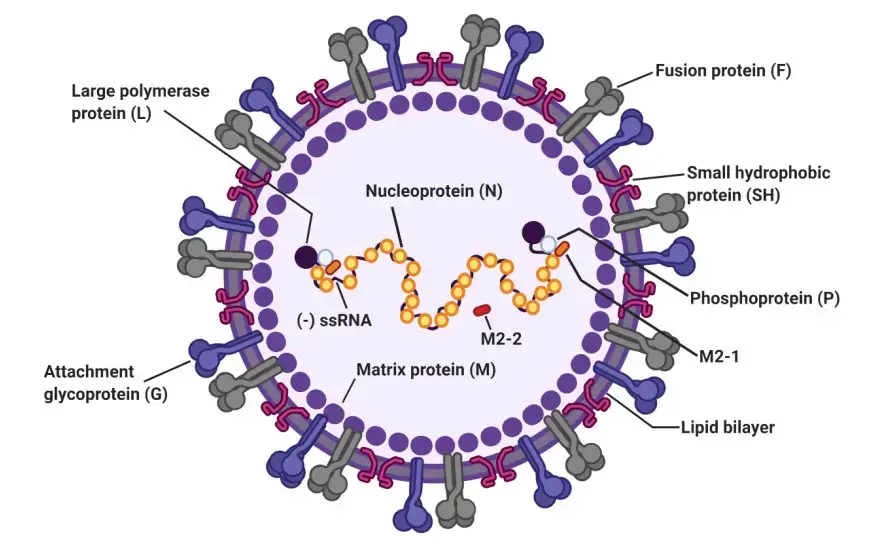
The RSV virus is made up of a nucleocapsid enclosed in a protective envelope made from the host’s membrane. When the virus is grown in cell cultures, it can appear as round particles between 100-350 nanometers in diameter, or as long filaments that are between 60-120 nanometers in diameter and up to 10 micrometers in length.
The virus envelope is made up of three different proteins: the fusion protein (F), the attachment protein (G), and a small hydrophobic protein (SH). These proteins form separate groups, which are presented on the virus’s surface as short spikes that are between 11-16 nanometers long.
The matrix protein (M) is located under the virus’s protective envelope. The virus’s genetic material consists of single-stranded, negative-sense RNA, which is enclosed by the nucleoprotein (N). The RNA polymerase protein (L), phosphoprotein (P), and the transcription processivity factor M2-1 also remain associated with the nucleocapsid.
Genome Structure of Respiratory Syncytial Virus (RSV)
Genome Structure of Respiratory Syncytial Virus (RSV)
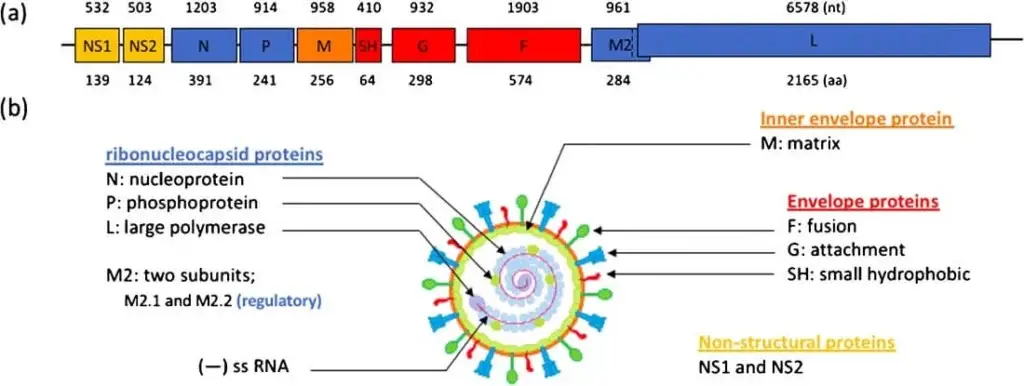
- The genetic material of the RSV virus is made up of a single-stranded, negative-sense RNA that contains 10 open reading frames (ORFs) and is approximately 15.2 kilobases in size. This RNA is responsible for encoding a total of 11 different proteins, both structural and non-structural.
- In order for RNA replication to occur, a complementary copy of the genome called the antigenome is involved. Neither the genome nor the antigenome contain 5′ caps or 3′ polyA tails.
- The RNA consists of two distinct regions located at the 3′ and 5′ ends, respectively. The leader region is located at the 3′ end and the trailer region is located at the 5′ end. The RNA is organized in a specific order, with NS1, NS2, N, P, M, SH, G, F, M2, and L following the leader region, and the trailer region at the end.
- The RNA is protected from degradation by encapsidation, which also helps to shield it from recognition by the host cell’s receptors that would otherwise trigger an immune response.
Epidemiology of Respiratory Syncytial Virus (RSV)
- In 1901, respiratory syncytial virus (RSV) was initially referred to as “acute catarrhal bronchitis.” It was isolated in 1956 and is responsible for 45%–90% of bronchiolitis cases, 15%–35% of pneumonia, 6%–8% of croup, as well as apnea and middle ear infections.
- By their first birthday, more than fifty percent of children are infected. 2 More than 80% of children have been infected with RSV at least once by the age of 2, and half of these children have been infected twice.
- In 2000, RSV caused 1.7 million physician office visits, 402,000 emergency room visits, 236,000 hospital outpatient visits, and 86,000 hospitalizations of children younger than 5 years old.
- In the United States, the treatment of RSV was estimated to cost $394 million for hospitalizations and $258 million for other medical encounters. Another study investigating four years of data estimated the emergency room costs of RSV in the United States to be approximately $202 million and the total hospital costs of RSV as a primary diagnosis to exceed $2.6 billion.
- One to three of every 100 patients with RSV as the primary infection are hospitalized, the majority of whom are infants aged 2 to 6 months.15% of patients diagnosed with RSV are intubated.
- 1 From 1979 to 1997, the Centers for Disease Control and Prevention (CDC) determined that 200 to 500 children perish annually from RSV-associated illnesses.
- Another study examining mortality in 1999 discovered 390 infant fatalities with probable RSV association. In the normal population, mortality is typically low (1%). Patients with chronic respiratory disease, immunodeficiency, or cardiovascular disease have a 3-5% mortality risk.
- From 1988 to 1991, a Canadian study found a 1% mortality rate among children hospitalized with RSV. In this study, infants with underlying lung or cardiac disease had an increased risk of death.
- RSV affects not only infants but also adults. Some studies indicate that the annual incidence of RSV in adults is comparable to that of influenza A.
- Each year, RSV infects 3%–7% of the elderly and 4%–10% of individuals at high risk. RSV infects healthy adults with upper respiratory symptoms such as cough, rhinorrhea, and congestion. Each year, however, RSV causes approximately 170,000 hospitalizations and 10,000 fatalities in patients older than 65.
- Additionally, it has been estimated that RSV is responsible for 17 out of every 1000 nursing facility resident deaths. RSV disease and outcomes have predominantly focused on children, but studies of new medications and vaccines should also evaluate adults, particularly the elderly population.
Transmission of Respiratory Syncytial Virus (RSV)
- Respiratory Syncytial Virus (RSV) can be transmitted through contact with respiratory secretions, such as saliva, mucus, or nasal secretions, from infected individuals. This can occur through close contact with an infected person, such as touching or shaking hands, or through contact with contaminated surfaces or objects.
- The virus can also be spread through the air when an infected person coughs or sneezes, releasing respiratory droplets containing the virus into the environment. These droplets can then be inhaled by others, particularly in crowded settings or enclosed spaces.
- Infants and young children are particularly vulnerable to RSV infection and can easily acquire it through contact with contaminated surfaces or by being in close contact with an infected person. Individuals with weakened immune systems or chronic medical conditions may also be at increased risk for severe RSV infections.
- Preventive measures, such as frequent hand washing, avoiding close contact with infected individuals, and disinfecting frequently touched surfaces, can help reduce the risk of RSV transmission. Vaccines and medications may also be available for preventing or treating RSV infection in certain high-risk populations.
Replication of Respiratory Syncytial Virus (RSV)
- RSV initiates the infection cycle by interacting with a receptor on the cell surface and fusing its envelope with the plasma membrane. This process transports the nucleocapsid into the cytoplasm of the cell.
- The nucleocapsid is comprised of the viral genome RNA, encapsulated along its length with the nucleoprotein (N) to form a helical structure, and associated with the viral RNA-dependent polymerase, a complex consisting of the viral large polymerase subunit (L), phosphoprotein (P), and.
- Once the nucleocapsid has entered the cytoplasm, the polymerase transcribes the viral mRNAs and replicates the genome by producing a positive-sense RNA intermediate, the antigenome, which serves as a template for subsequent genome RNA synthesis.
- As newly synthesized genomes (and antigenomes) are synthesized, they become encapsulated with N protein and bind to polymerase proteins to form nucleocapsids. Nucleocapsids are transported to the plasma membrane, where they associate with other viral structural proteins, and virions are released through budding.
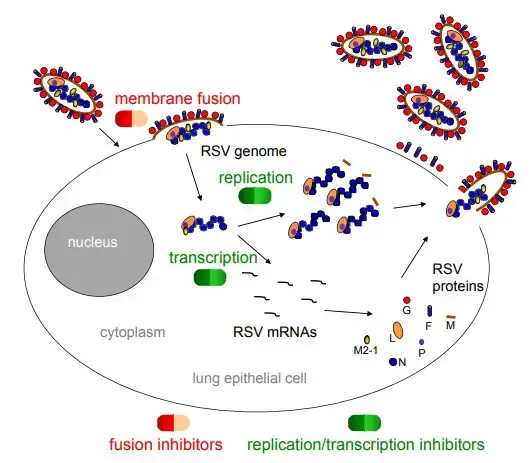
- The attachment glycoprotein (G protein, red circle) binds to the chemokine receptor, CX3CR1, on the apical surface of ciliated epithelial cells, and the fusion protein (F protein, purple cylinder) facilitates entrance of the nucleocapsid into the cytoplasm.
- The N-RNA complex (blue circle chain) functions as a template for the RSV polymerase complex, composed of the L and P proteins (orange and purple ovals, respectively), to synthesize mRNAs and progeny genomes. M2-1 protein is required for transcription as well (yellow circle).
- The surface glycoproteins are synthesized, modified post-translationally, and transported along the endoplasmic reticulum and exocytic pathway. The matrix (M) protein (brown rod) associates with replication complexes in the cytoplasm and interacts with the cytoplasmic tail of the F protein to induce viral filament formation.
- This allows nucleocapsids to be packaged into filamentous virus particles, which are discharged from the cell plasma membrane by bursting. In addition to the proteins described above and depicted in the figure, RSV also produces two nonstructural proteins, NS1 and NS2, which inhibit the innate immune response to infection.
- By analogy with the closely related human metapneumovirus, RSV also expresses M2-2, a negative regulator of transcription that may serve to increase polymerase fidelity.
- The SH protein is a viroporin that influences membrane permeability. The RSV life cycle stages that are targeted by fusion and replication/translation inhibitors are highlighted.
Pathogenesis of Respiratory Syncytial Virus (RSV)
- Typically, RSV is restricted to the respiratory tract and lymphocytic peribronchiolar infiltration is observed. RSV replicates in the nasopharynx and causes cell necrosis by binding to the bronchiolar epithelium.
- Hypersecretion of mucus and infiltration of round cells cause edema in the surrounding submucosa. Thus, the narrow airways’ lumina become obstructed.
- Hyperinflation is the result of air becoming confined in the periphery, and atelectasis is the result of the trapped air being absorbed.
- Interleukins, chemokines, and leukotrienes are released during and after an infection, causing inflammation and tissue injury.
- The epithelium begins to heal during the first week of illness, whereas the ciliated cells do not recover until several weeks later.
Clinical Manifestations of Respiratory Syncytial Virus (RSV)
Respiratory Syncytial Virus (RSV) can cause a range of respiratory symptoms, ranging from mild to severe, particularly in infants and young children. Symptoms may include:
- Runny or stuffy nose
- Coughing
- Sneezing
- Sore throat
- Mild fever
- Wheezing
- Difficulty breathing
- Rapid breathing
- Cyanosis (bluish skin color due to lack of oxygen)
In some cases, RSV infection can lead to more serious complications, such as pneumonia, bronchiolitis (inflammation of the small airways in the lungs), or even respiratory failure. Infants and young children, as well as individuals with weakened immune systems or underlying medical conditions, may be at increased risk for severe RSV infection.
Treatment for RSV infection typically focuses on managing symptoms, such as using fever-reducing medications, maintaining hydration, and providing supplemental oxygen or respiratory support, as needed. In severe cases, hospitalization may be necessary. Preemptive measures, such as good hygiene practices and vaccination, may also help reduce the risk of RSV infection and its complications.
Diagnosis of Respiratory Syncytial Virus (RSV)
- Rapid Antigen Detection Tests (RADTs) are popular for RSV testing due to ease of use, quick turnaround time, and acceptable sensitivity and specificity.
- RADTs can sometimes provide false-negative results, so a more sensitive method like PCR should be considered in those cases.
- Direct Fluorescent Antibody (DFA) is a reliable method for infants and young children for RSV diagnosis, but shows poorer sensitivity in adults due to lower viral shedding rates compared to younger children.
- Polymerase Chain Reaction (PCR) is a highly sensitive and specific method for detecting RSV in nasopharyngeal swabs and aspirates. It is preferred for older children and adults, and for hospitalized and immunocompromised patients. However, it is more expensive than DFA and may have a longer turnaround time in certain laboratory settings.
- Viral culture is not recommended for initial clinical management due to a slow turnaround time of about 3-5 days and lower sensitivity. However, it is important for the detection of coinfections, has high specificity, and the virus can be stored for diagnostic studies.
- Other laboratory tests used to diagnose RSV complications include blood tests to examine white cell counts or detect viruses, bacteria, and other germs, chest X-rays to check for lung inflammation, and pulse oximetry (using a painless skin monitor) to detect lower than normal oxygen levels in the blood.
Treatment of Respiratory Syncytial Virus (RSV)
Currently, there are no specific antiviral medications available for the treatment of Respiratory Syncytial Virus (RSV). Treatment is generally supportive and aimed at relieving symptoms.
- For mild cases, treatment may include rest, hydration, and fever-reducing medications (e.g. acetaminophen).
- For more severe cases, hospitalization may be required for oxygen therapy, mechanical ventilation, and other supportive measures.
- In some cases, antiviral medications such as ribavirin or palivizumab may be considered, particularly in high-risk patients such as premature infants or those with underlying medical conditions. However, the use of these medications is controversial and their effectiveness is still under debate.
- Corticosteroids are not recommended for routine use in the treatment of RSV due to potential adverse effects and limited benefit.
- Prevention strategies such as good hygiene practices, hand washing, and avoiding contact with infected individuals may help reduce the risk of RSV infection. Vaccines for RSV are available but only for certain populations (e.g. infants at high risk of severe RSV disease).
Prevention and Control of Respiratory Syncytial Virus (RSV)
Prevention and control of Respiratory Syncytial Virus (RSV) mainly involves implementing measures to reduce the risk of transmission, particularly among high-risk individuals. These may include:
- Hand hygiene: Regular hand washing with soap and water or alcohol-based hand sanitizers can help reduce the spread of RSV.
- Avoiding contact with sick individuals: Individuals with RSV should avoid close contact with others, particularly those who are at high risk of severe RSV disease, such as infants, older adults, and those with weakened immune systems.
- Covering coughs and sneezes: Coughing or sneezing into a tissue or the crook of the elbow can help prevent the spread of respiratory viruses like RSV.
- Cleaning and disinfecting frequently touched surfaces: Surfaces such as door handles, countertops, and toys should be regularly cleaned and disinfected, particularly during RSV season.
- Respiratory etiquette: Individuals with RSV should wear a face mask or cover their nose and mouth with a tissue when around others.
- Vaccination: Vaccines are available for certain populations, such as infants at high risk of severe RSV disease.
- Prophylaxis: Palivizumab, a monoclonal antibody, can be given to high-risk infants and young children during RSV season to prevent severe RSV disease.
- Isolation and cohorting: In healthcare settings, infected individuals may be isolated and grouped together (cohorting) to reduce the risk of transmission to others.
- Education: Education campaigns aimed at promoting awareness of RSV and its transmission can help individuals take appropriate precautions to reduce the risk of infection.
FAQ
What is Respiratory Syncytial Virus (RSV)?
Respiratory Syncytial Virus (RSV) is a highly contagious virus that can cause respiratory infections in people of all ages, but is most common in young children and infants.
What are the symptoms of RSV infection?
Symptoms of RSV infection can include coughing, sneezing, fever, difficulty breathing, wheezing, and a runny or stuffy nose.
How is RSV transmitted?
RSV is primarily transmitted through close contact with an infected person, especially through contact with respiratory secretions like saliva or mucus.
Who is most at risk for severe RSV infection?
Infants, young children, and people with weakened immune systems or underlying health conditions are most at risk for severe RSV infection.
Can RSV infection be prevented?
The spread of RSV infection can be prevented by practicing good hand hygiene, avoiding close contact with infected individuals, and keeping surfaces clean and disinfected.
Is there a vaccine for RSV?
While there is no vaccine currently available for RSV, research is ongoing and there are treatments available for people with severe cases of the infection.
How is RSV diagnosed?
RSV infection can be diagnosed through a variety of methods, including rapid antigen detection tests, direct fluorescent antibody tests, and polymerase chain reaction tests.
What are the treatment options for RSV infection?
Treatment options for RSV infection can include rest, hydration, and over-the-counter medications to manage symptoms, as well as antiviral medications for more severe cases.
Can RSV infection be fatal?
In rare cases, RSV infection can be fatal, especially in young children, infants, and people with weakened immune systems.
How long does it take to recover from RSV infection?
Recovery time from RSV infection can vary depending on the severity of the infection and the individual’s overall health, but most people recover within one to two weeks.
References
- Taleb, S.A., Al Thani, A.A., Al Ansari, K. et al. Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches. Eur J Clin Microbiol Infect Dis 37, 1817–1827 (2018). https://doi.org/10.1007/s10096-018-3289-4
- Fearns, Rachel & Deval, Jerome. (2016). New antiviral approaches for respiratory syncytial virus and other mononegaviruses: Inhibiting the RNA polymerase. Antiviral research. 134. 10.1016/j.antiviral.2016.08.006.
- Rocca, Alessandro & Biagi, Carlotta & Scarpini, Sara & Dondi, Arianna & Vandini, Silvia & Pierantoni, Luca & Lanari, Marcello. (2021). Passive Immunoprophylaxis against Respiratory Syncytial Virus in Children: Where Are We Now?. International Journal of Molecular Sciences. 22. 3703. 10.3390/ijms22073703.
- Azzari, Chiara & Baraldi, Eugenio & Bonanni, Paolo & Bozzola, Elena & Coscia, Alessandra & Lanari, Marcello & Manzoni, Paolo & Mazzone, Teresa & Sandri, Fabrizio & Lisi, Giovanni & Parisi, Salvatore & Piacentini, Giorgio & Mosca, Fabio. (2021). Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Italian Journal of Pediatrics. 47. 198. 10.1186/s13052-021-01148-8.
- Jung, & Kim, & Lee,. (2020). Contribution of Dendritic Cells in Protective Immunity against Respiratory Syncytial Virus Infection. Viruses. 12. 102. 10.3390/v12010102.
- Kiss G, Holl JM, Williams GM, Alonas E, Vanover D, Lifland AW, Gudheti M, Guerrero-Ferreira RC, Nair V, Yi H, Graham BS, Santangelo PJ, Wright ER. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J Virol. 2014 Jul;88(13):7602-17. doi: 10.1128/JVI.00256-14. Epub 2014 Apr 23. PMID: 24760890; PMCID: PMC4054448.
- Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol. 2013;372:3-38. doi: 10.1007/978-3-642-38919-1_1. PMID: 24362682; PMCID: PMC4794264.
- Eiland LS. Respiratory syncytial virus: diagnosis, treatment and prevention. J Pediatr Pharmacol Ther. 2009 Apr;14(2):75-85. doi: 10.5863/1551-6776-14.2.75. PMID: 23055894; PMCID: PMC3461981.
- https://www.niaid.nih.gov/diseases-conditions/respiratory-syncytial-virus-rsv
- https://www.lung.org/lung-health-diseases/lung-disease-lookup/rsv
- https://www.lung.org/lung-health-diseases/lung-disease-lookup/rsv/learn-about-rsv
- https://www.mayoclinic.org/diseases-conditions/respiratory-syncytial-virus/symptoms-causes/syc-20353098
- https://www.cdc.gov/rsv/index.html
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.