Glycolysis is the primary stage in the process of breaking down glucose in order to obtain energy to power the cell’s metabolism. The majority of living organisms perform glycolysis as a part in their metabolic process. This process does not require oxygen, which is why it is considered anaerobic. Glycolysis happens in the cytoplasms of both eukaryotic as well as prokaryotic cells.
The process of introducing glucose into heterotrophic cells takes place via two different ways. One is through secondary active transport where the transport happens against the gradient of glucose concentration. Another method is to use an array of integral proteins known as GLUT proteins, which are also known by the name glucose transporter proteins. These transporters are involved in facilitation of the transport of glucose.
Regulation of glycolysis Introduction
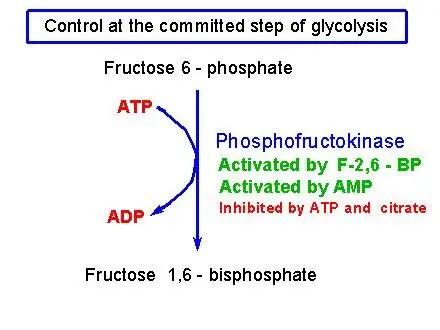
A variety of glycolysis processes are controlled, but the main and most significant control point is the 3rd stage of the process that is catalyzed by an enzyme known as the phosphofructokinase (PFK). This is the first stage that is committed, making it a major goal for the regulation of the glycolysis process in general.
The PFK gene is controlled by ATP which is an ADP derivative known as AMP and citrate along with other molecules we’ll not talk about here.
- ATP: ATP is a negative regulator of PFK that makes sense that if there’s plenty of ATP within cells, then glycolysis will not require the production of more.
- AMP: Adenosine monophosphate (AMP) is an important regulator of PFK. When cells are low in ATP and AMP, it begins making more ATP from ADP molecules, turning them into ATP as well as AMP (ADP + ADP Right-right Arrow ATP + AMP). A high level of AMP means that cells are starved to eat, and glycolysis has to be running quickly in order to replenish the ATP.
- Citrate: Citrate, which is the initial product from the citric acid process, is also a potent inhibitor of PFK. If citrate is accumulating it is an indication that glycolysis may slow down, as it is in a state of overflow and does not require more fuel.
Regulation of glycolysis
The enzymes are the primary components that power the metabolic pathway. Hence investigating the mechanisms that regulate these enzymes will provide insight into the processes that affect glycolysis. There are nine main steps in glycolysis, which is governed by 14 different enzymes. The enzymes can be altered or affected by 5 principal regulatory processes, such as post-translational modification (PTM) as well as localization.
1. Biological mechanisms by which enzymes are regulated
There are five biological mechanisms that allow enzymes to be controlled such as:
- Gene Expression
- Allostery
- Protein-protein interaction (PPI)
- Post translational modification (PTM)
- Localization
2. Regulation by insulin in animals
In animals, the regulation in blood sugar levels through the pancreas combination along with the liver are an essential component of homeostasis. Beta cells that reside in the pancreatic islets can be sensitive to the level of blood glucose.
A rise in blood glucose levels triggers them to release insulin into the bloodstream and has an impact specifically on the liver however, it also affects muscle and fat cells, causing them to take glucose out of the blood. If blood sugar levels drop, those pancreas beta cells stop production of insulin, but instead, they stimulate neighboring pancreatic alpha cells , which release glucagon into blood.
This results in your liver to expel glucose from bloodstream by breaking down glycogen stored through the process of gluconeogenesis. If the drop in blood glucose levels is especially intense or sudden the other glucose sensors trigger an increase in epinephrine out of the adrenal glands to the blood. It has the same effect similar to glucagon in regulating glucose metabolism, however its effects are more prominent.
In the liver, glucagon as well as epinephrine trigger the phosphorylation of the most important rate-limiting enzymes of glycolysis and fatty acid synthesis cholesterol production, gluconeogenesis and glycogenolysis. Insulin exerts a negative effect upon these enzymes.
The phosphorylation and dephosphorylation process of the enzymes (ultimately as a result of glucose levels in blood) is the predominant method that this pathway is controlled within the liver as well as in muscle cells. The phosphorylation of phosphofructokinase blocks glycolysis and its dephosphorylation by an insulin-like action triggers glycolysis.
3. Regulation of the rate limiting enzymes
The four enzymes responsible for regulation include the hexokinase (or glucokinase found in the liver) along with phosphofructokinase as well as the pyruvate kinase. The flow of glycolysis pathway is regulated according to the conditions within and outside of the cell. The internal elements which regulate glycolysis work mostly to provide ATP in sufficient quantities to meet the cells’ needs. External factors act principally upon the fat tissues, liver and muscles, and can take large amounts of glucose from blood following meals (thus keeping out hyperglycemia by storage of excess glucose in glycogen or glycogen, based on the type of tissue). The liver also has the capability at releasing glucose to blood after meals, during the fasting period, and while exercising, which prevents hypoglycemia via the use of glycogenolysis and the process of gluconeogenesis. These reactions are correlated with the stoppage of glycolysis within the liver.
Additionally, glucokinase and hexokinase are independent of hormonal influences to regulate the entry points for glucose into cells in various tissues. Hexokinase reacts with the glucose-6-phosphate (G6P) level within the cell or, for glucosekinase, to blood sugar levels in blood, thereby allowing for the complete intracellular controls for the glycolytic pathway within various organs (see the section below).
Once glucose has been transformed to G6P through glucokinase or hexokinase the glucose can be converted into glucose-1-phosphate (G1P) for conversion into glycogen, or transformed by glycolysis into Pyruvate. It then is transported to the mitochondrion, where it is transformed into acetyl-CoA, and later into citrate.
The excess citrate is emitted from the mitochondrion to the cytosol, from where ATP citrate lyase re-creates acetyl CoA and Oxaloacetate (OAA). Acetyl-CoA is used for the synthesis of fatty acids as well as cholesterol synthesis, two crucial methods of using excess glucose when it can be high within blood. The enzymes with the lowest rate catalyzing these reactions are activated after they have been dephosphorylated as a result of the insulin action on liver cells. When you eat, fast or exercise, or during hypoglycemia the hormones epinephrine and glucagon are released into blood.
The liver glycogen is then able to be converted back into G6P which is then converted into glucose through the liver-specific enzyme glucose 6-phosphatase , which is released into blood. Glucagon and epinephrine are also responsible for the process of gluconeogenesis. It converts carbohydrates that are not carbohydrate-based into G6P that joins G6P made from glycogen or acts as a substitute for it when the glycogen stores in the liver stores have been depleted.
This is crucial to brain function, as the brain relies on glucose as a source of energy in the majority of circumstances. The simultaneous phosphorylation, specifically, phosphofructokinase but in addition, to some extent , pyruvate Kinase, stops glycolysis from occurring in tandem with glycogenolysis and gluconeogenesis.
a. Hexokinase and glucokinase
- Cells contain the enzyme hexokinase. It helps convert glucose that has been introduced into cells into glucose-6-phosphate (G6P). Since the cell’s membrane is insensitive to G6P, hexokinase functions to transfer glucose into cells, from which it will be unable to escape. Hexokinase is blocked by the large levels of G6P within the cell. Therefore, the speed at which glucose is absorbed into cells partly depends on the speed at which G6P can be removed by glycolysis, as well as by glycogen synthesizing (in the cells that store glycogen, specifically the muscles and the liver).
- It is the only enzyme that, unlike hexokinase is not inhibited by the G6P. It is found in the liver cells and can only phosphorylate glucose that enters the cell , resulting in glucose-6-phosphate (G6P) when the glucose in blood is plentiful. This is the initial step of the glycolytic pathway within the liver provides an additional layer of control to the glycolytic process in the liver.


b. Phosphofructokinase
Phosphofructokinase plays a significant control element in the glycolytic pathway since it is among the irreversible processes and contains significant allosteric effectsors, such as fructose 2,6 -bisphosphate (F2,6BP).
2.6% of Fructose (F2,6BP) has been identified as a powerful activator of the phosphofructokinase (PFK-1) which is produced when F6P is phosphorylated an additional the phosphofructokinase (PFK2). In the liver when blood sugar levels are low and glucagon raises the level of cAMP, PFK2 gets activated by protein kinase. The phosphorylation deactivates PFK2 and a second domain of the protein activates as fructose bisphosphatase-2. This enzyme transforms F2,6BP back into F6P. Both epinephrine as well as glucagon result in an increase in cAMP levels in the liver.
The result of lower levels of liver fructose-2,6-bisphosphate is a decrease in activity of phosphofructokinase and an increase in activity of fructose 1,6-bisphosphatase, so that gluconeogenesis (in essence, “glycolysis in reverse”) is favored. This is in line with the function that the liver plays in these circumstances, since the reaction from the liver’s hormones to the hormones’ release is glucose into the blood.
ATP is competing against AMP to form the effector location in the PFK enzyme. ATP is present in the cells significantly more than AMP generally 100-fold higher however, the amount of ATP doesn’t change by over a period of more than 10% in the conditions of physiology, while an increase of 10% in ATP is accompanied by a 6-fold rise in AMP. This suggests that the importance to ATP in the role of an allosteric influencing agent is in doubt. The increase in AMP is a result of a reduction in the energy charge of the cell.
Citrate blocks phosphofructokinase in vitro. It does this by enhancing the inhibitory effects of ATP. However, it’s not certain that this has significant in vivo since citrate is used primarily for conversion to the acetyl-CoA form for fatty acid as well as cholesterol synthesizing.
TIGAR is a p53 inducible enzyme is responsible for controlling phosphofructokinase. It is able to guard against the effects of oxidative stress. TIGAR is an enzyme that has a dual functions that regulate F2,6BP. It can behave as a phosphatase (fructuose-2,6-bisphosphatase) which cleaves the phosphate at carbon-2 producing F6P. It also functions as an Kinase (PFK2) by putting an phosphate to carbon-2 of F6P, which results in F2,6BP. In humans it is known that the TIGAR protein is encoded in the C12orf5 gene. The TIGAR enzyme can hinder the progression of glycolysis by causing an accumulation of fructose-6-phosphate (F6P) that is then isomerized to glucose-6-phosphate (G6P). As the accumulation increases, G6P will cause carbons to be sucked into an phosphate pentose pathway.
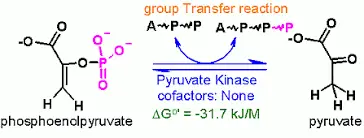
c. Pyruvate kinase
Pyruvate kinase enzyme is responsible for the final step of glycolysis during which pyruvate as well as ATP are created. Pyruvate kinase is responsible for an exchange between aphosphate group the phosphoenolpyruvate (PEP) and ADP and produces 1 molecule of pyruvate, and one molecule of the ATP.
Liver pyruvate kinase can be controlled by glucagon and epinephrine via protein kinase. This protein kinase phosphorylates the liver the pyruvate kinase, which is then deactivated. The pyruvate kinase in the muscle is not affected by the activation of epinephrine in protein kinase. Glucagon signals fasting (no glucose in the body).
This is why glycolysis is impeded within the liver but is not affected in muscles during you are fasting. A rise in blood sugar results in production of insulin that stimulates phosphoproteinphosphatase I which leads to dephosphorylation, and then activation of the pyruvate kinase. This prevents pyruvate kinase from activating simultaneously with enzymes responsible for catalyzing an opposite reactions (pyruvate carboxylase, and phosphoenolpyruvate carbonoxykinas) which prevents a futile cycle.
References
- https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/CHEM_4320_5320%3A_Biochemistry_1/9%3A_Glycolysis_and_Gluconeogenesis/9.1%3A_Glycolysis_-_Reaction_and_Regulation
- https://www.slideshare.net/YESANNA/glycolysis-42953283
- http://guweb2.gonzaga.edu/faculty/cronk/CHEM245pub/glycolysis-regulation.html
- https://www.khanacademy.org/science/biology/cellular-respiration-and-fermentation/variations-on-cellular-respiration/a/regulation-of-cellular-respiration
- https://en.wikipedia.org/wiki/Glycolysis#Regulation
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.