What is Radioimmunoassay (RIA)?
- The radioimmunoassay (RIA) technique is used to evaluate the presence and concentration of antigens or antibodies in a material. It is an in vitro approach, which means it occurs outside of a living body.
- The basic idea of RIA is to use radioisotopes as labels that are coupled with antigens or antibodies. Instead of enzymes, radioisotopes are used as detection markers. This approach enables exact measurement of the antigen-antibody complex with high sensitivity.
- RIA was developed by Solomon Berson and Rosalyn Yalow of the Veterans Administration Hospital in New York, who initially described it in 1960. RIA was initially used to assess endogenous plasma insulin levels.
- Competitive binding underpins traditional RIA methodologies. An unlabeled antigen competes with a radiolabeled antigen for binding to a particular antibody in this method. When a mixture of radiolabeled and unlabeled antigens is incubated with the matching antibody, the amount of free radiolabeled antigen (not bound to the antibody) present in the mixture is exactly proportional to the amount of unlabeled antigen present.
- The concentration of the unlabeled antigen in the sample can be determined by measuring the radioactivity of the free radiolabeled antigen. The more unlabeled antigen there is, the more it competes with radiolabeled antigen, resulting in a decrease in the amount of free radiolabeled antigen.
- There are various advantages of using RIA over other immunoassay procedures. It is extremely sensitive, capable of identifying minute amounts of antigens or antibodies in a sample. This sensitivity is important in a variety of applications, including clinical diagnostics and medical research. RIA is also well-known for its specificity, which allows for precise measurement of target compounds even in complicated biological samples.
- However, because of the possible risks connected with radioactivity, the use of radioisotopes in RIA necessitates additional measures. As a result, non-radioactive labeling techniques such as enzyme-linked immunosorbent test (ELISA) have been developed.
- In conclusion, radioimmunoassay (RIA) is a strong immunoassay technique that uses radioisotopes as labels to detect and quantify antigens or antibodies. It is a highly sensitive approach based on competitive binding that allows for precise measurements of target compounds in a variety of research and diagnostic applications.
Dfinition of Radioimmunoassay (RIA)
Radioimmunoassay (RIA) is a highly sensitive laboratory technique that uses radioisotopes to detect and measure the concentration of antigens or antibodies in a sample.
Radioimmunoassay (RIA) Principle
The principle of radioimmunoassay (RIA) is based on competitive binding, specificity, and sensitivity.
In RIA, a radioactive antigen, also known as a “tracer,” competes with a non-radioactive antigen (from standards or samples) for a limited number of binding sites on antibodies or receptors. The binding between antigens and antibodies is specific, forming the antigen-antibody complex.
The unlabeled antigens present in the sample compete with the radiolabeled antigens to bind to the specific antibodies. As the concentration of unlabeled antigens increases, they replace the labeled antigens that were initially bound to the antibodies. Consequently, the amount of free radiolabeled antigens in the solution increases. Thus, the concentration of the bound unlabeled antigens is directly proportional to the concentration of the free labeled antigens.
The three key principles involved in RIA are:
- Immune reaction: The binding of antigens and antibodies to form the antigen-antibody complex. This step is specific to the target antigen.
- Competitive binding or competitive displacement reaction: The unlabeled antigens from the sample compete with the labeled antigens for binding to the specific antibodies. This competitive interaction provides specificity, as only the target antigen displaces the labeled antigen.
- Measurement of radio emission: The radioisotopes conjugated to the antigens emit radioactivity, which can be measured. The concentration of the free labeled antigens is directly related to the amount of bound unlabeled antigens. By measuring the radioactivity, the concentration of the target antigen in the sample can be determined. This step provides the high sensitivity of RIA, allowing for the detection of very low concentrations of the target analyte.
Overall, the RIA principle relies on the competitive binding of labeled and unlabeled antigens to antibodies, which can be quantitatively measured based on the emitted radioactivity, providing a sensitive and specific method for antigen detection and quantification.
Immune Reaction
- The immune reaction is a vital process in the body’s immune system, triggered when a foreign biological substance enters the bloodstream through a non-oral route. The body recognizes the unique chemical characteristics on the surface of the foreign substance and identifies it as an antigen. In response, the immune system produces specific antibodies that are designed to neutralize the effects of the antigen and protect the body.
- The immune reaction involves the interaction between antibodies and antigens. Antibodies are proteins produced by specialized immune cells called B lymphocytes. These antibodies have a specific structure that allows them to bind to the corresponding antigen with high specificity. The binding of antibodies to antigens forms an antigen-antibody complex.
- Unlike the principle of electrophoresis, where proteins are separated based on their charge, the movement of antibodies or antigens in the immune reaction is influenced by chemical interactions. The binding between antibodies and antigens is a result of molecular recognition and complementary shapes, allowing for specific and selective binding.
- The immune reaction is a highly regulated and complex process that plays a crucial role in defending the body against pathogens, toxins, and other foreign substances. By producing specific antibodies against antigens, the immune system can identify and neutralize these threats, preventing harm to the body.
- Overall, the immune reaction involves the production of antibodies by the immune system in response to the presence of foreign antigens. The binding and movement of antibodies and antigens are driven by chemical interactions, enabling the immune system to effectively recognize and neutralize harmful substances.
Competitive binding or competitive displacement reaction
- Competitive binding or competitive displacement reaction is a phenomenon observed when multiple antigens have the potential to bind to the same antibody. In this scenario, the antigen with a higher concentration will exhibit more extensive binding to the limited antibody, effectively displacing other antigens from binding sites.
- In an experimental setup utilizing competitive binding, a radiolabeled antigen is initially allowed to bind to a high-affinity antibody. This labeled antigen serves as a tracer and facilitates the detection of antigen-antibody complexes. However, when patient serum or a sample containing unlabeled antigens is introduced, these unlabeled antigens begin to compete for binding sites on the antibody, thereby displacing the labeled antigen.
- The displacement of the labeled antigen occurs because the unlabeled antigens present in the patient serum have a higher concentration or affinity for the antibody compared to the labeled antigen. As a result, the unlabeled antigens effectively “compete” with the labeled antigen for binding, leading to the displacement of the labeled antigen from the antibody binding sites.
- The displacement of the labeled antigen by unlabeled antigens can be quantified by measuring the decrease in the amount of radiolabeled antigen bound to the antibody. This displacement reflects the concentration or presence of the unlabeled antigen in the sample.
- Competitive binding or competitive displacement reactions are commonly employed in various immunoassay techniques, including radioimmunoassay (RIA), to determine the concentration of specific antigens in a sample. By assessing the extent of displacement, the concentration of the unlabeled antigen can be inferred, providing valuable information for diagnostic and research purposes.
Measurement of radio emission
- The measurement of radio emission is a crucial step in radioimmunoassay (RIA) and contributes to its high sensitivity. After the incubation period, where the labeled antigen competes with unlabeled antigens from the sample for binding to specific antibodies, the next step involves washing to remove any unbound antigens.
- Following the washing step, the radio emission of the antigen-antibody complex is measured. This is done by detecting the gamma rays emitted by the radiolabeled antigen. The radiolabeled antigen serves as a marker, allowing for the quantification of the bound antigen.
- The measurement of radio emission provides a means to determine the concentration of the target antigen in the patient’s serum. The competition between labeled and unlabeled antigens for the limited number of specific antibodies results in the release of a certain amount of labeled antigen. This amount is directly proportional to the ratio of labeled to unlabeled antigens in the sample. By measuring the radioactivity of the free antigens remaining in the supernatant, the concentration of the target antigen can be derived.
- To quantify the antigen concentration accurately, a binding curve is constructed. This involves plotting the percentage of antibody-bound radiolabeled antigen against known concentrations of a standardized unlabeled antigen. From this curve, the concentration of antigen in patient samples can be extrapolated.
- The measurement of radio emission in RIA enables highly sensitive detection and quantification of antigens. The ability to detect low concentrations of antigens, sometimes reaching the level of picograms, is one of the major advantages of RIA. This sensitivity is essential in various applications, such as clinical diagnostics and medical research, where accurate and precise measurement of antigen levels is crucial.
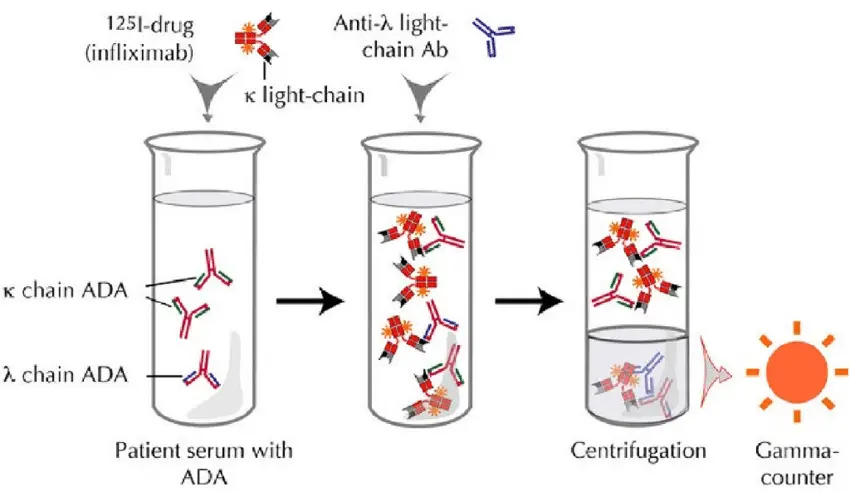
Requirements for Radioimmunoassay (RIA)
Radioimmunoassay (RIA) requires several key components and materials to perform the assay effectively. The following are the essential requirements for RIA:
- Radiolabeled antigens: The antigens used in RIA are labeled with radioactive isotopes. Commonly used isotopes include gamma-ray emitting isotopes like I-125 and beta-ray emitting isotopes like Tritium. These labeled antigens are often referred to as “hot antigens” because of their radioactive nature. The radioactive label enables the measurement of radioactivity and allows for the quantification of antigen-antibody interactions.
- Unlabeled antigens (sample antigens): In addition to the radiolabeled antigens, unlabeled antigens are required for competitive binding in RIA. These unlabeled antigens, also known as “cold antigens,” are derived from the samples being tested. They compete with the radiolabeled antigens for binding to the specific antibodies. The concentration of these unlabeled antigens in the sample is of interest for quantitative analysis.
- Specific antibodies: Specific antibodies are necessary to bind with the target antigens in the assay. These antibodies should have high affinity and specificity towards the target antigen. They are used in smaller amounts compared to the antigens. The antibodies recognize and bind to the antigens, forming antigen-antibody complexes that can be measured.
- Washing buffer solutions: To remove unbound antigens and antibodies and reduce non-specific binding, washing steps are crucial in RIA. Wash buffer solutions are used to wash the microtitre plate wells after each incubation step. A common wash buffer used in RIA is a solution of 1% Trifluoroacetic acid, which helps to minimize non-specific interactions and ensure accurate measurements.
- Microtitre plates: RIA is often performed using microtitre plates, specifically the 96-well format. These plates have multiple wells, each capable of holding a small volume of the assay mixture. The wells allow for simultaneous testing of multiple samples and standards, increasing efficiency and throughput.
Equipment required
Performing a radioimmunoassay (RIA) requires specific equipment to handle the samples, perform the necessary manipulations, and measure the radioactivity. The following are some of the essential equipment typically used in RIA:
- Pipettors and/or pipets: Accurate and precise delivery of required volumes of reagents and samples is crucial in RIA. Pipettors or pipets of appropriate sizes are used to ensure precise measurement and transfer of liquid volumes.
- Polypropylene test tubes: RIA involves multiple incubation and separation steps. Polypropylene test tubes are commonly used as they are chemically resistant and can withstand the necessary centrifugation and mixing steps.
- Test tube rack: A test tube rack provides a convenient and organized way to hold and handle the test tubes during the assay steps.
- Beakers or flasks: Beakers or flasks of suitable sizes are used for the preparation of reagents, dilutions, and other solutions required during the RIA procedure.
- Vortex mixer: A vortex mixer is used for efficient mixing and resuspension of reagents and samples. It ensures thorough and consistent mixing of the components.
- Centrifuge: A refrigerated centrifuge with a swinging bucket rotor is commonly used in RIA. The centrifuge enables the separation of bound and unbound components by applying centrifugal force. The refrigeration feature helps maintain sample integrity and stability during the process.
- Liquid scintillation counter or gamma counter: These instruments are used to measure the radioactivity in the RIA. A liquid scintillation counter, such as a Tri-Carb® or MicroBeta™ liquid scintillation counter, or a gamma counter, such as a Wizard2 gamma counter, is used to measure the emitted radiation from the radiolabeled antigens or complexes. These instruments provide accurate quantification of radioactivity, allowing the calculation of antigen concentrations.
These equipment items, including pipettors, polypropylene test tubes, test tube rack, beakers or flasks, vortex mixer, centrifuge, and appropriate radioactivity measurement instruments, are essential for performing RIA accurately and efficiently. They enable precise handling of reagents and samples and facilitate the measurement of radioactivity, which is fundamental to the success of the assay.
Radioimmunoassay (RIA) Protocol
The radioimmunoassay (RIA) protocol involves several steps to accurately measure the concentration of a specific antigen using radiolabeled and unlabeled antigens. Here is a general outline of the RIA protocol:
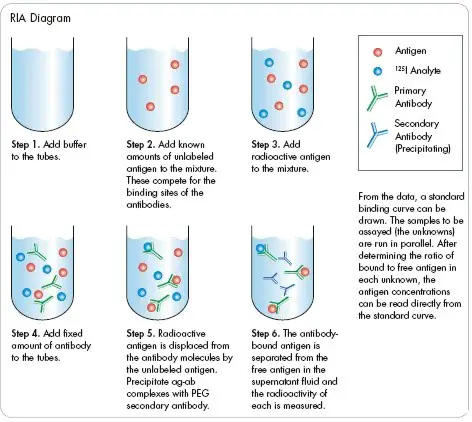
- Preparation of microtitre plate: Specific antibodies of known concentration are fixed or immobilized onto the wells of a microtitre plate. This ensures that the antibodies are available to interact with the antigens during the assay.
- Addition of radiolabeled antigens: A known amount of radiolabeled or “hot” antigens is added to each well of the microtitre plate. The radiolabeled antigens have been previously labeled with a radioactive isotope, allowing for their detection and measurement.
- Washing step: The microtitre plate is carefully washed to remove any unbound antigens that did not bind to the antibodies. This step helps reduce non-specific binding and ensures accurate measurements.
- Measurement of maximum radioactivity: After washing, the radioactivity of the microtitre plate wells is measured using a gamma-counter. At this point, the radioactivity will be at its maximum level since the radiolabeled antigens that have bound to the antibodies contribute to the overall radioactivity.
- Addition of unlabeled antigens: Unlabeled antigens, also known as “cold” antigens, are added to each well. These unlabeled antigens compete with the radiolabeled antigens for binding sites on the antibodies.
- Washing step: The microtitre plate is washed again to remove any free radiolabeled antigens that were displaced by the unlabeled antigens. Washing ensures that only the bound antigens remain in the wells.
- Measurement of remaining radioactivity: The radioactivity of the microtitre plate wells is measured once more using a gamma-counter. This measurement reflects the remaining radioactivity after the displacement of radiolabeled antigens by the unlabeled antigens.
By comparing the radioactivity measured before and after the addition of unlabeled antigens, the concentration of unlabeled antigens in the sample can be determined.
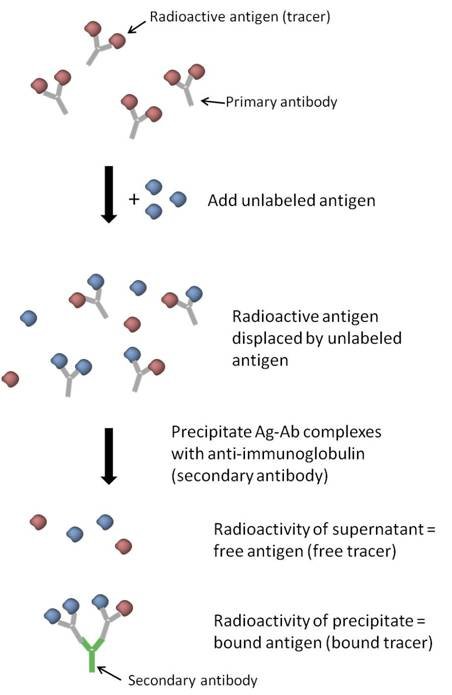
The RIA protocol described above follows the principle of competitive binding, where the unlabeled antigens compete with the radiolabeled antigens for binding to the specific antibodies. The amount of radiolabeled antigen displaced by the unlabeled antigen is indicative of the concentration of the unlabeled antigen in the sample. This competitive displacement allows for the quantitative measurement of antigens in the RIA.
Result Interpretation of Radioimmunoassay (RIA)
The interpretation of radioimmunoassay (RIA) results involves analyzing the changes in radioactivity to determine the presence and concentration of the antigen of interest. Here is a summary of the result interpretation process in RIA:
- Maximum radioactivity: Initially, when the radiolabeled antigens are added to the assay, they bind to the specific antibodies present in the wells, resulting in maximum radioactivity. This serves as the reference point for subsequent measurements.
- Decrease in radioactivity: If the sample being tested contains the specific antigens of interest, they will compete with the radiolabeled antigens and bind to the antibodies, displacing the labeled antigens. As a result, the radioactivity of the solution decreases compared to the maximum level. This decrease indicates the presence of the antigen in the sample.
- No change in radioactivity: If the radioactivity remains the same after the addition of the sample, it suggests that the antigen of interest is not present in the sample. This indicates a negative result for the antigen being tested.
- Construction of a standard curve: To determine the concentration of the antigen in the sample, a standard curve is generated. This is achieved by plotting a graph of the radioactivity (expressed as a percentage of the maximum) against the concentration of the unlabeled antigens used in the assay. The standard curve represents the relationship between the concentration of the unlabeled antigens and the corresponding decrease in radioactivity.
- Calibration of sample radioactivity: The sample being assayed is run in parallel with the standards, following a similar procedure. The radioactivity measured in the sample is then compared to the standard curve. By interpolating or extrapolating the radioactivity value on the standard curve, the concentration of the antigen in the sample can be determined.
By interpreting the changes in radioactivity and comparing them to the standard curve, RIA allows for the quantitative measurement of antigens in the sample. The decrease in radioactivity indicates the presence of the antigen, while the calibration with the standard curve enables the determination of its concentration.
Applications of Radioimmunoassay (RIA)
Radioimmunoassay (RIA) has found wide applications in various fields due to its high sensitivity and specificity. Here are some notable applications of RIA:
- Detection of peptide hormones: RIA was initially developed for the detection of peptide hormones. It has been extensively used to measure the concentration of hormones such as insulin, growth hormone, thyroid-stimulating hormone (TSH), and many others in biological samples.
- Detection of viral antigens: RIA has been employed in the detection of viral antigens, aiding in the diagnosis and monitoring of viral infections. It has been used to detect specific viral antigens associated with diseases like HIV, hepatitis viruses, and respiratory viruses.
- Measurement of hormones and drugs: RIA is widely utilized for the quantification of hormones and drugs in biological samples. It has been instrumental in monitoring drug levels in therapeutic drug monitoring, assessing hormonal imbalances, and determining drug abuse.
- Detection of Hepatitis B surface antigens: RIA has played a crucial role in the detection of Hepatitis B surface antigens, aiding in the diagnosis and management of Hepatitis B virus infections.
- Detection of mycotoxins: RIA has been utilized in the detection of mycotoxins, which are toxic substances produced by fungi. By using specific antibodies against mycotoxins, RIA enables sensitive and accurate detection of these harmful substances in food and environmental samples.
- Early-stage cancer detection: RIA has shown promise in the early detection of certain types of cancer. By measuring specific tumor markers or antigens in blood or tissue samples, RIA can assist in identifying cancer at its early stages when treatment options are often more effective.
These applications highlight the versatility of RIA in diverse areas, including endocrinology, virology, toxicology, and oncology. The high sensitivity and specificity of RIA make it a valuable tool for quantitative measurement and detection of various analytes, contributing to advancements in medical diagnosis, research, and public health.
Calculations
To calculate the concentrations of analyte in the samples in a Radioimmunoassay (RIA), the following steps are typically followed:
- Average the counts for each set of tube replicates to obtain the average counts per minute (CPM).
- Calculate the average net counts for all standards and samples by subtracting the average non-specific binding counts from each.
- Determine the normalized percent bound (% B/B0) for each standard and sample using the formula provided in the content.
- Plot the % B/B0 for each standard against the corresponding amounts of unlabeled ligand calibrator added in picograms to create a standard curve.
- Interpolate the amount of analyte in each sample from the standard curve. The sample values should be corrected for any dilutions performed during the assay to determine the original concentration in the sample.
- If any samples have concentrations above the range of the standard curve, they should be diluted with assay buffer and re-assayed. The values obtained should be multiplied by the appropriate dilution factor.
% B/B0 = Net cpm of Standard or Sample x 100/ Net cpm of “0” Standard
It’s important to note that values obtained from the assay that are lower than the first calibration standard may be suspect.
In the provided example (Table), the raw and processed data for the assay are presented. The tube numbers, counts per minute (CPM), average CPM, net average CPM, and % B/B0 values are given. The standard curve can be constructed using the % B/B0 values and the corresponding amounts of unlabeled ligand calibrator. The concentration of analyte in the sample (Sample #21 and Sample #22) can then be determined by interpolating from the standard curve.
a.
| Tube | No. | CPM | Avg CPM | Net Avg CPM | %B/B0 |
|---|---|---|---|---|---|
| Total Counts | 1 | 14963 | |||
| 2 | 15563 | 15263 | |||
| Blank | 3 | 415 | |||
| 4 | 380 | 398 | |||
| “0” Standard | 5 | 8879 | |||
| 6 | 8673 | 8776 | 8378 | 100 | |
| 2 pg | 7 | 7701 | |||
| 8 | 7939 | 7820 | 7422 | 88.6 | |
| 5 pg | 9 | 6873 | |||
| 10 | 6886 | 6880 | 6482 | 77.4 | |
| 10 pg | 11 | 5632 | |||
| 12 | 5900 | 5766 | 5368 | 64.1 | |
| 25 pg | 13 | 4216 | |||
| 14 | 4256 | 4236 | 3838 | 45.8 | |
| 50 pg | 15 | 3082 | |||
| 16 | 2995 | 3039 | 2641 | 31.5 | |
| 100 pg | 17 | 2257 | |||
| 18 | 2003 | 2130 | 1732 | 20.7 | |
| 200 pg | 19 | 1491 | |||
| 20 | 1661 | 1576 | 1178 | 14.1 | |
| Sample | 21 | 5829 | |||
| 22 | 5919 | 5874 | 5476 | 65.4 |
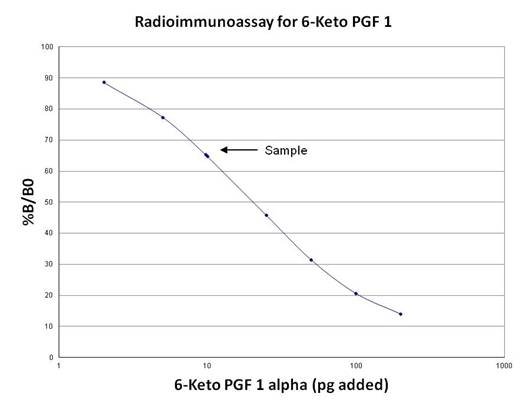
By following these calculations, the RIA provides a means to quantify the concentration of analytes in the samples based on the radioactivity measurements and the standard curve derived from known concentrations of the analyte.
Advantages Radioimmunoassay (RIA)
Radioimmunoassay (RIA) offers several advantages that make it a valuable technique in the field of biomedical research and diagnostics. Here are some of the advantages of RIA:
- High specificity: RIA exhibits a high level of specificity, allowing for the precise detection of target antigens or antibodies. The use of specific antibodies ensures that only the desired analyte is measured, reducing the chances of cross-reactivity or interference from other substances present in the sample.
- High sensitivity: RIA is known for its exceptional sensitivity. It can detect and quantify even very low concentrations of antigens or antibodies. RIA can measure concentrations in the range of picograms (10^-12 grams) or even femtograms (10^-15 grams), making it suitable for applications where detecting minute quantities of analytes is critical.
- Detection of small amounts: RIA can accurately measure nanogram (10^-9 grams) quantities of antigens or antibodies. This capability is particularly useful in situations where the analyte of interest is present in low concentrations or when dealing with limited sample volumes.
- Wide range of applications: RIA has been successfully applied in various fields, including endocrinology, virology, pharmacology, and toxicology. It has been extensively used for measuring hormones, detecting viral antigens, monitoring drug levels, and diagnosing diseases such as hepatitis. The versatility of RIA makes it a valuable tool in both research and clinical settings.
- Quantitative results: RIA provides quantitative results, allowing for the precise measurement of analyte concentrations. This quantitative data enables researchers and clinicians to assess the levels of antigens or antibodies in biological samples, monitor changes over time, and make informed decisions about patient care or experimental outcomes.
- Established technique: RIA is a well-established and widely recognized technique, with decades of successful applications and a wealth of literature supporting its use. It has undergone extensive validation and standardization, ensuring reliability and reproducibility of results across different laboratories.
Overall, the high specificity, sensitivity, and capability to detect small amounts of analytes make RIA a powerful tool in biomedical research and diagnostics. Its quantitative nature and broad range of applications contribute to its continued relevance and utility in various fields of study.
Limitations Radioimmunoassay (RIA)
Radioimmunoassay (RIA) has several limitations that need to be considered when using this technique. Here are some of the limitations of RIA:
- Radioactive materials and safety concerns: RIA involves the use of radioactive isotopes as labels for antigens or antibodies. Working with radioactive substances poses potential health risks to laboratory personnel, requiring strict adherence to safety protocols and precautions. Specialized facilities and equipment may be necessary to handle and store radioactive materials safely.
- Disposal of radioactive waste: Proper disposal of radioactive waste is a critical consideration in RIA. The disposal process must adhere to strict regulations and guidelines to prevent environmental contamination and ensure the safety of individuals handling the waste. Disposal procedures can be complex and may require specialized facilities and expertise.
- Cost of equipment and reagents: RIA requires specialized equipment, including liquid scintillation counters or gamma counters, as well as specific reagents and materials. These equipment and reagents can be expensive, making the initial setup and ongoing maintenance costs a limiting factor for some laboratories or research institutions.
- Shelf-life of radiolabeled substances: Radiolabeled substances used in RIA typically have a short shelf-life due to the decay of radioisotopes over time. This limited shelf-life necessitates careful management of stock solutions and frequent procurement or synthesis of fresh radiolabeled substances. It adds to the complexity and cost of maintaining a reliable supply of labeled antigens or antibodies.
- Regulatory requirements: The use of radioactive materials in RIA is subject to stringent regulatory requirements, including licensing and compliance with radiation safety regulations. Laboratories and researchers must meet these regulatory obligations, which can involve paperwork, monitoring, and regular inspections, adding administrative burdens and potential delays.
- Non-quantitative results: Although RIA provides quantitative measurements of analyte concentrations, it may not provide detailed information about other parameters, such as the functional activity or conformational changes of the analyte. Other techniques, such as enzyme-linked immunosorbent assay (ELISA) or Western blotting, may be more suitable for certain types of analyses requiring additional characterization.
It is essential to consider these limitations when deciding to utilize RIA. While RIA offers high sensitivity and specificity, the associated risks, costs, and regulatory requirements may necessitate careful planning, expertise, and adherence to safety protocols. Researchers should also explore alternative non-radioactive immunoassay techniques, such as enzyme-based or fluorescence-based assays, which offer similar specificity and sensitivity without the use of radioactive materials.
FAQ
What is Radioimmunoassay (RIA)?
Radioimmunoassay is a sensitive laboratory technique used to measure the concentration of specific antigens or antibodies in a sample using radioisotopes as labels.
What are the applications of RIA?
RIA has various applications, including the detection of hormones, viral antigens, drugs, mycotoxins, and early-stage cancer. It has been widely used in medical diagnosis, research, and pharmaceutical development.
Is RIA a sensitive method?
Yes, RIA is known for its high sensitivity. It can detect very low concentrations of antigens or antibodies, typically in the nanogram range. This makes it useful for measuring substances present in small quantities.
What are the advantages of RIA?
The advantages of RIA include its high specificity, high sensitivity, and the ability to measure small amounts of analytes. It has been a valuable tool in medical and scientific fields for accurate and precise measurement of substances of interest.
Are there any limitations or drawbacks to RIA?
RIA does have some limitations. It involves working with radioactive substances, which can pose safety risks and require proper handling and disposal procedures. The equipment and reagents used in RIA can be expensive, and radiolabeled substances have a short shelf-life, requiring frequent preparation.
How does RIA work?
RIA works based on the principle of competitive binding. A radiolabeled antigen competes with a non-radioactive antigen in the sample for binding to a limited number of specific antibodies. The amount of radiolabeled antigen bound to the antibody is inversely proportional to the concentration of the unlabeled antigen in the sample.
Is RIA widely used in clinical laboratories?
RIA was widely used in clinical laboratories in the past but has been largely replaced by non-radioactive immunoassay methods, such as enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (CLIA). These alternative methods offer similar sensitivity and specificity without the need for radioisotopes.
How long does an RIA test take?
The duration of an RIA test can vary depending on the specific assay and laboratory protocols. It typically involves several steps, including incubation, washing, and radioactivity measurement. The overall process can range from a few hours to a full day.
Is RIA still used in research settings?
RIA is still used in certain research settings where its high sensitivity is required or when studying specific substances that are better detected using radioisotopes. However, non-radioactive immunoassay methods have become more popular due to safety concerns and advances in technology.
Can RIA be performed by non-specialists?
RIA requires specialized knowledge and training in handling radioactive materials and operating the necessary equipment. It is typically performed by trained laboratory professionals who have experience in radioisotope handling and immunoassay techniques.
References
- Radioimmunoassay. W.M. Hunter in Handbook of Experimental Immunology-Volume 1 Immunochemistry, D.M. Weir (Ed), Blackwell Scientific Publications, London 1978.
- Radioimmunoassay. K.E. Kirkham & W. M. Hunter, Williams & Wilkins Co., Baltimore, MD 1971
- Personal Communications- S. Richard Harris Ph.D, Dupont-NEN Biomedical Products.
- Personal Communications- David Handfield and Scott Keohane, Perkin Elmer Life & Analytical Sciences.
- Immunology – Dr Janis Kuby – W.H. Freeman & Co., NY 2007
- Parija S.C., (2009), Textbook of Microbiology and Immunology, 2nd edition, Elsevier, a division of Reed Elsevier India Private Limited, pg. 113-114.
- Goldsby R.A., Kindt T.J., Osborne B.A., (1999) Kuby Immunology, 4th edition, W.H.Freeman & Co Ltd., pg. 147-148.
- Goldsmith SJ. Radioimmunoassay: review of basic principles. Semin Nucl Med. 1975 Apr;5(2):125-52. DOI: 10.1016/s0001-2998(75)80028-6. PMID: 164695.
- Howell-Moroney, Madeleine, “Radioimmunoassay”. Embryo Project Encyclopedia (2022-01-24). ISSN: 1940-5030 http://embryo.asu.edu/handle/10776/13324.