What is Post-transcriptional Modification?
Post-transcriptional modification (PTM) are changes that occur after RNA has been made but before it is converted into protein. These changes are mainly seen in eukaryotic cells, in the nucleus, and right after transcription. The RNA that is just made is called pre-mRNA (or hnRNA), and it is not yet ready for translation.
In PTM, the RNA is converted into a form that is both stable and functional. A special 5’ cap and a poly(A) tail are added to the RNA, and also the introns are removed through a splicing process. The exons (the parts of the genes which code for proteins) are the only ones that are joined together to form mature mRNA. Sometimes, a single gene can produce different mRNAs depending on the splicing pattern used, which is known as alternative splicing.
The 5’ cap (a methylated guanosine) works as a protective shield for RNA against enzymes that degrade it, and it also helps ribosome to locate the starting point of translation. A poly(A) tail is added at the 3’ end, instead of the 5’. It consists of many adenine nucleotides chained one after another. The tail is also an RNA stabilizer in cytoplasm and a transporter from nucleus.
The PTM step is not limited to splicing, tailing, and capping only. At times, RNA editing is happening — where a few nucleotides are replaced, or added, or even deleted. Such a tiny change may quite significantly alter the protein that is going to be synthesized.
Besides that, there are chemical changes on RNA bases, for instance, methylation, which may influence RNA properties or its life-span. Thus, PTM can be seen as a control system that dictates timing, location, and quantity of the protein that will be made from the particular RNA.
Without the RNA processing that is part of PTM, many RNAs would be short-lived and wrongly translated. Therefore, the entire PTM mechanism not only makes gene expression more efficient but also more flexible — it allows the same genetic code to generate a greater number of proteins.
Definition of Post-transcriptional Modification
Post-transcriptional modification refers to the chemical alterations and changes that occur to RNA molecules after they have been transcribed from DNA. These modifications can include processes such as alternative splicing, capping, polyadenylation, and RNA editing, which collectively regulate gene expression and contribute to the diversity of proteins produced in a cell.
Location of Post-transcriptional Modification
Most of these post-transcriptional changes of the mRNA take place in the nucleus of the eukaryotic cell. Splitting out, capping, and polyadenylation of the RNA are the steps necessary to be accomplished before the newly transcribed RNA can be called mature and leave the nucleus. First of all, almost all of the reaction pathways start in this very organelle and frequently they are merged operations that run on the still existing RNA transcript.
Removal of introns from the pre-mRNA and the ligation of exons by the spliceosome complex happens in nuclear RNA splicing. The cutting of the RNA can be done while the RNA polymerase is still on the gene or very shortly after the transcription is finished. Nuclear speckles or dots are the terms for the areas in the nucleoplasm where splicing factors are located and where RNA splicing comes to a concentration.
The 5’ end capping is done almost immediately after the RNA is around 25–30 nucleotides long. The methylated 7-methylguanosine cap is attached almost the moment the RNA polymerase II moves on from that short stretch, thus the modification location is nuclear and not post-export.
The 3’ polyadenylation (and cleavage) of the RNA is a nuclear event as well. A specific sequence — AAUAAA — serves as a signal for an enzyme where to make the cut and also from where the long poly(A) tail is to be added. This change occurs prior to the RNA polymerase actually finishing transcription.
Nucleus is the principal location for RNA editing such as A-to-I conversion as well, since this is where most of the ADAR enzymes can be found. Some RNAs, especially those extensively edited at the Alu regions, may be left deliberately entrapped in the nuclear compartment by protein complexes to prevent them from early release.
The other extensions to the RNA also explode in the cell nucleus-consequently, the very first trimming of microRNA (miRNA) intermediates and the shaping of long noncoding RNAs (lncRNAs). The RNAs are mostly retained in the nucleus until they have been processed enough to be functional.
Once the mature RNA goes through these early steps, it is transported to the cytoplasm where the regulation takes place at a later stage. At this level, the control of RNA stability and of translation is carried out. miRNAs bind to target RNAs, the enzyme Dicer cleaves them into short fragments that associate with the RISC complex thus, the RISC complex silences the mRNAs that have identical sequences.
There are times when cytoplasmic polyadenylation happens — such is a case of oocyte maturation when expired maternal mRNAs are once again given poly(A) tails in order to initiate translation. Moreover, an isoform of ADAR (ADAR1 p150) which shuttles between nucleus and cytoplasm, alters some RNA sequences right in the cytosol.
Unlike in eukaryotic cells, the situation in prokaryotes is totally different. Due to the absence of a nucleus, these processes take place simultaneously in the cytoplasm. The mRNA is spared from extensive processing, but meanwhile, rRNA and tRNA undergo minor trimming and base modification steps before acquiring their functionality.
Importance of Post-transcriptional modifications
- Post-transcriptional modification (PTM) is still the most crucial stage in the whole life cycle of an RNA molecule as it ensures that the RNA is mature and fit for protein production. A stage without PTM will cause the resulting product of transcription (the pre-mRNA) to be unrecognizable to ribosomes, thus leading to an incomplete translation or wrong proteins being produced.
- First of all, PTM is a process that gives the RNA the necessary tools to defend itself against the degrading enzymes. Both the 5′ cap and the poly(A) tail are parts of the RNA that are being protected. While the cap is protecting the 5′ end of the RNA from nuclease attack, the tail keeps it safe from the 3′ end. That little thing is what actually changes the RNA’s lifetime in the cytoplasm, which indirectly leads to an increase in protein production.
- By splicing, the removal of introns is performed so that only the correct coding part is left. This is important because the translation machinery will not be wasting time on the useless sequences. Another point is that RNA can be spliced in various ways (alternative splicing) to create different proteins from a single gene. This is the main reason why eukaryotes can produce a vast number of proteins from a limited number of genes.
- PTM provide cellular machinery with an additional level of genetic control. A good example is the case of RNA base editing (adenosine to inosine), which can affect the protein amino acid sequence or can regulate the RNA localization in the cell. Hence, even after transcription, the cell still has the power to control what the end product will be — this adaptability is very common in tissue-specific expression.
- RNA editing along with methylation modifications are also aspects of cellular adaptation. Certain modifications cause RNA to fold differently or to interact with proteins differently. These minor chemical changes can sometimes determine the stability of the RNA or the rate at which it is translated and thus they have a significant effect on gene expression indirectly.
- On occasion, PTM is employed as a means to regulate the timing of gene expression. A good example is the cytoplasmic polyadenylation during oocyte maturation which generates the activation of the stored mRNAs at the precise time. This slow translation is a way of ensuring that the proteins will be there only at the moment when the cell needs them.
Types of post-translational modification
1. Phosphorylation – addition of phosphate (PO₄³⁻) group usually to serine, threonine, or tyrosine residues. It was catalyzed by enzymes called kinases, and reversed by phosphatases. This modification control many signaling pathways and enzyme activity in cells.
2. Glycosylation – attachment of carbohydrate chains to proteins for proper folding and stability. Two major types exist – N-linked (attached to amide nitrogen of asparagine) and O-linked (to hydroxyl group of serine/threonine). Occurs mainly in endoplasmic reticulum (ER) and Golgi apparatus, and it affect protein trafficking / recognition.
3. Acetylation – in this process acetyl group (–COCH₃) added to the amino group of lysine residues or N-terminal. Histone acetylation controls gene expression by loosening DNA–histone interaction – so transcription becomes more active. Sometimes deacetylation done by HDACs (histone deacetylases).
4. Methylation – here a methyl group added to nitrogen or oxygen atoms of amino acid side chains. Usually found in histone proteins, changing chromatin structure and gene silencing. One or more methyls can be added (mon-, di-, tri-), and effects depend on site / context.
5. Ubiquitination – attachment of small protein ubiquitin (≈ 8.5 kDa) to lysine residue of target protein. Marks them for degradation by proteasome 26S, but sometimes it also change location or activity instead of destroy them.
6. Lipidation – lipid molecule like palmitate, myristate, or prenyl group added to protein. This anchoring makes protein associate with membrane. Example – palmitoylation on G-proteins helps in signaling.
7. Hydroxylation – hydroxyl group (–OH) added mainly to proline and lysine residues in collagen. It was needed for stabilization of the triple-helix structure. Requires vitamin C, so its deficiency cause scurvy (due to weak collagen).
8. Sumoylation – similar to ubiquitin but with SUMO (small ubiquitin-like modifier). It regulate nuclear transport, transcription factors, and protein stability. Sumo conjugation and deconjugation is reversible.
9. Disulfide Bond Formation – oxidation between two cysteine residues forming disulfide bridge (–S–S–). Provide stability to protein folding especially in extracellular proteins. Sometimes they reshuffle during folding for correct structure.
10. Proteolytic Cleavage – peptide chains cut by specific proteases to activate or mature the protein. For example, insulin is formed after cleavage of proinsulin chain. This type of modification is irreversible, so once done it cannot be prevail back.
Post-transcriptional modification Steps
The following modifications occur during the Post-transcriptional modification of proteins;
Addition of 5′ cap
5’ End Capping – this modification happens on the nascent mRNA soon after it comes out from RNA Polymerase II (RNAP II) about 20–30 nucleotides long. The cap, made of 7-methylguanosine (m⁷G), attach to the first nucleotide at the 5’ end.
The process goes by three steps, each done by different enzymes.
- RNA triphosphatase remove the 5’-terminal triphosphate group — leaving a diphosphate end ready for next reaction.
- Then capping enzyme catalyze guanylation using GTP, forming that unusual 5’–5’ backward linkage (it’s kind of flipped, compared to normal 3’–5’ bonds).
- At last, guanine-7-methyltransferase add methyl (–CH₃) to the new guanine, completing the cap structure.
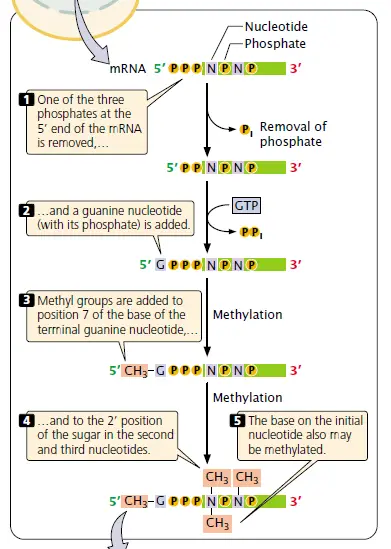
After that, the cap protect mRNA from exonuclease attack / degradation and also help them bind with cap-binding complex (CBC). It act like passport for transport by nucleus → cytoplasm, so the mature mRNA can reach ribosome for translation. Some people forget that capping also helps in splicing and initiation of translation etc.
This reaction, though enzymatically simple, decide stability and recognition of RNA molecules – without it they get degraded fast and no protein is made properly.
Importance of Addition of 5’ End Capping
- Importance of Addition of 5’ End Capping – mRNA was protected from rapid exonuclease digestion by the m⁷G cap, so transcripts survive long enough for processing and translation; without the cap, messages are degraded quickly, and protein output is lost.
- Recognition for export – the cap is bound by the cap-binding complex (CBC) which is required for nuclear export; the mRNA is thus flagged as RNAP II product, and then it is escorted out of nucleus to cytoplasm, otherwise the transcript often remain trapped or are retained and degraded.
- Initiation of translation – the cap structure was required for efficient recruitment of translation initiation factors, the ribosome assembly is promoted by cap recognition, hence correct start codon selection is enabled; translation initiation is impaired when cap absent.
- mRNA processing coordination – splicing and 3′-end processing are influenced by the cap, because the early capped transcript is engaged by processing machineries, therefore splicing efficiency and correct exon joining are increased, faults are reduced.
- Protection vs innate immunity / surveillance – capped RNAs are discriminated from uncapped or aberrant RNAs by surveillance systems, and decapping or incorrect cap leads to recognition by decay pathways; the cap also help to reduce mistaken activation of innate antiviral sensors in cytoplasm.
- Regulation of stability and localization – cap modifications (like additional methylations) modulate half-life, localization, and translation rates, so mRNA fate is determined in part by cap status — many regulatory decisions are thereby influenced.
- Quality-control signal – the cap acts as an early quality marker indicating proper RNAP II transcription and processing; faulty or missing cap often lead to immediate degradation, so only properly matured transcripts are used for translation.
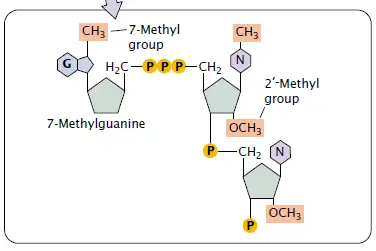
The Addition of the Poly A tail – 3’-OH, polyadenylation
The 3’-OH end of pre-mRNA can be compared to a free handle, where new nucleotides can be attached.
One protein complex that is bound to the RNA polymerase II CTD tail is responsible for cutting the 3’ region close to an AAUAAA sequence, thus creating a short overhang with that 3’-OH ready.
The step in which the cut end is covered with a series of adenines is called polyadenylation.
It is the enzyme poly(A) polymerase (PAP) that carries out the addition but PAP on its own has a weak affinity for RNA, so CPSF (cleavage and polyadenylation specificity factor) is the one that helps it to stay there.
Also CF I and CF II factors are involved, though the enzyme which makes the first cut has not yet been definitely identified.
The one that grows, called poly(A) tail, can be of a length of 200–250 nt etc., the exact length being regulated by poly(A) binding protein II (PABII).
Being in the nucleus PABII is the one that keeps the chain going smoothly up to a certain size — after that it stops PAP from adding more bases.
mRNA is exported to cytoplasm, where a different protein named PABP (poly(A) binding protein) that binds the same tail and somewhat protects it by covering those A’s from nuclease attack.
With time poly(A) tail gets shorter ; when it is very close to being gone , mRNA becomes unstable and will be degraded shortly.
Therefore , longer tails are often interpreted as longer mRNA lifetime in cytoplasm, but no direct correlation has been found by scientists and they believe it’s more complex than that.
Poly(A) tail is actually not the main factor of translation, on the contrary, it just allows RNA to be around for a longer time thus more proteins can be synthesized before it disappears.
Basically –polyadenylation is the process of attaching adenine nucleotides to 3’-OH end by PAP with the help of CPSF, CFI, CFII, and PABII, thus ensuring both the finishing and the stability of the transcript.
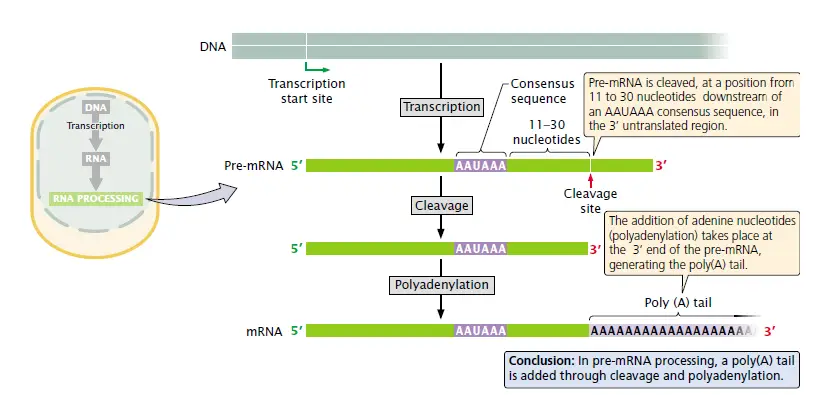
RNA Splicing
In eukaryotic cells, the RNA produced by RNA polymerase II cannot be used for translation directly as it has introns and exons combined together.
RNA splicing is the process where introns are removed, and exons are linked at 3’-OH ends. This process takes place mainly in the nucleus before RNA is transported out.
The 3’-OH group on the exon serves as the reactive site; it binds to the phosphate at the intron–exon junction, thus breaking the bond and resulting in the joining of the neighboring exon.
RNA splicing involves two transesterification reactions — firstly, the branchpoint adenine (A) of the intron forms a loop by binding its 2’-OH to the 5’ splice site.
Subsequently, the free 3’-OH on the upstream exon interacts with the site again; this reaction releases the intron in a lariat form (a small looped RNA) and joins the two exons together.
The entire operation is facilitated by a large complex known as a spliceosome which is a combination of proteins and several snRNAs (small nuclear RNAs) of about 100–200 bases in length.
The different parts of a spliceosome such as U1, U2, U4/U6, and U5 snRNPs not only identify the splice sites but also facilitate rna folding and carry out the chemistry nearly by themselves.
The introns are degraded quickly after they are removed; the exons are ligated to form a mature mRNA that then moves through nuclear pores to the cytoplasm, and so on.
In a number of genes, splicing can also be performed alternatively thus different exons combinations can be generated – that is the alternative splicing.
This means that a single gene can produce multiple mRNA variants / different proteins (e.g. antibody chains).
There are only two special forms – trans-splicing which links exons from different pre-mRNAs and the second one which radically changes protein structure by removing whole exons.
Sometimes the very RNA structure is involved in catalysis ; there are a few ribozymes that can splice themselves without any protein, just by their own folded conformation.
Among the most important features ensuring accuracy are the 5’ splice site, branch point, and 3’ splice site – if any of these are mis-recognized, incorrect mRNA will result.
Therefore RNA splicing is a 3’-OH chemistry and spliceosome work coordination ; it is the mechanism that achieves the transition from raw pre-mRNA to clean mRNA sequence readable by the ribosome.
Spliceosome
- The spliceosome is a large RNA–protein complex where the splicing of pre-mRNA, in fact, takes place in the nucleus.
- It consists mainly of snRNPs (small nuclear ribonucleoproteins) along with many other helper proteins / factors etc.
- Each snRNP consists of one snRNA component and several proteins; the RNA part hybridizes with regions on the pre-mRNA in order to locate the introns accurately.
- Typical snRNPs called U1, U2, U4/U6, U5, they associate and dissociate in a regulated manner during the splicing stages.
- Firstly U1 snRNP binds to 5’ splice site – the consensus region like AG|GUAAGU – and thus delineates the start of intron.
- U2 snRNP attaches at the branch-point adenine (A) but not directly on it ; hence the mismatch, the A protrudes as a small bulge ready for the incision.
- After that U4/U6 and U5 get together to form the active spliceosome core, however, U4 is already gone when the chemical reaction is initiated.
- The very first transesterification is performed when the 2’-OH of the branch A attacks the 5’-phosphate of intron ; with this the loop termed lariat is created.
- Without a doubt the 5’ exon is released but nevertheless it is kept tightly bound to U5, which holds the both exons so that they can react further without delay.
- The 3’-OH of the first exon performs the nucleophilic attack on the 5’ phosphate of the second exon in the second step of the reaction – this creates a phosphodiester bond between the two exons and lets go of the lariat intron.
- Spare lariat RNA is shortly degraded , thus mature mRNA with continuous coding region is left behind.
- Besides that U2AF (binds polypyrimidine tract) and SF1/BPP (binds branch-point region) are some of the factors that assist in proper positioning of U2 snRNP.
- Furthermore proteins from SR-family and hnRNP group regulate the spliceosome assembly as well as the strength of the splice sites.
- Different splice sites utilization is the cause of alternative splicing – stronger or weaker matches determine whether intron will be retained or removed ; thus the possibility of one gene to produce many proteins.
- The RNA conformation within spliceosome is surprisingly close to the self-splicing ribozymes – hence nature recycle the same structural principle again.
- Hence the spliceosome can be viewed as a versatile contraption – half RNA half protein – employing 3’-OH chemistry to ligate exons and excise introns in an orderly manner.
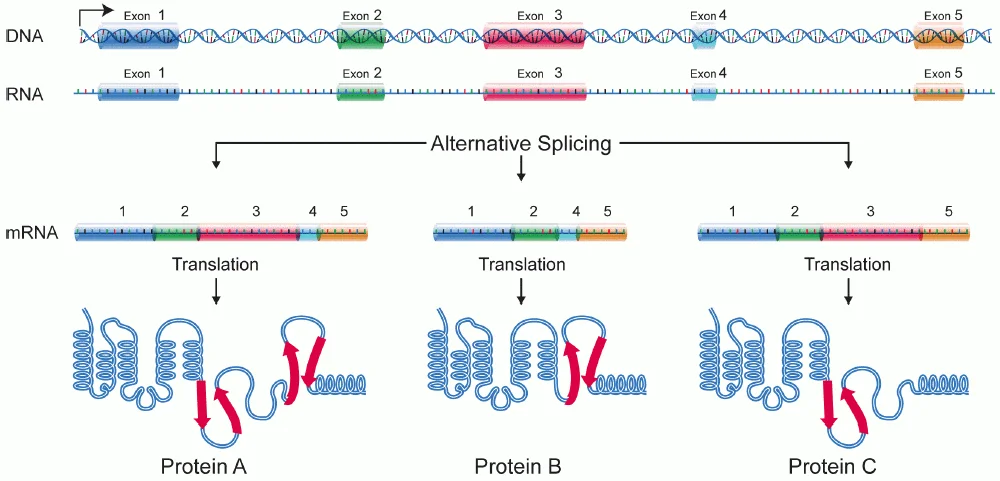
Alternate Splicing
- Alternative splicing refers to the process whereby the pre-mRNA produced from a single gene can be differently cut and ligated.
- Some exons are retained, and some may be skipped — i.e., it depends on the cell type or tissue where the transcript comes from.
- Therefore, a single gene sometimes can produce multiple proteins, such as 5–7 or even more, that are slightly different in their functions.
- This diversity arises from the spliceosome selecting different splice sites in the same transcription product.
- The presence of certain splicing factors in a given tissue determines which exons will be ligated, thus muscle cells splicing differently than neurons.
- For instance, the α-tropomyosin gene in humans is spliced differently in heart, smooth muscle, and non-muscle cells.
- Each mRNA variant generated from that gene codes for a related protein, but not the identical one – small differences in the sequence drastically change the protein’s behavior.
- Alternative patterns are mainly determined by the interaction of SR-proteins, hnRNPs, and other factors that bind near splice sites thus either exposing or hiding them.
- At times, an intron may remain as a coding region; at other times, a whole exon may be removed entirely – both resulting in the formation of new amino acid sequences.
- The rule of thumb is – identical DNA, different mRNAs, thus more proteins can be generated from the same genome, a kind of genome efficiency trick.
- In more complex organisms, this regulation is the main reason why the limited number of genes can still produce a huge variety of proteins.
- Alternative splicing is also sensitive to signals and stress; therefore, the same gene may respond differently under a new condition and rapidly generate a variant form.
- Even though the process is complicated, it still employs the 3’-OH chemistry and spliceosome mechanism as in regular splicing — only the site selection is different.
Significance of Post-transcriptional Modification
- After transcription, the pre-mRNA in eukaryotes is not directly convertible into protein; it requires a few repairing changes first.
- These changes are known as post-transcriptional modifications, and most of them take place within the nucleus before RNA is transported out.
- The 5′ end is given a methyl-guanosine cap; the cap not only shields mRNA from degradation but also facilitates ribosome binding later on.
- The 3′ end is getting poly(A) tail added by poly(A) polymerase, the tail making the message stable and determining the longevity of the message in the cytoplasm.
- While the work is going on, RNA splicing is also performed – introns are removed, and exons are joined together creating mature coding region.
- These stages in concert make the transcript stable, recognizable and more efficient for translation.
- Without the cap or tail, RNA would be very quickly degraded by exonucleases and the like; thus, the modification essentially rescues it.
- Occasionally RNA editing may also be taking place, where a few nucleotides are changed after synthesis, thus allowing slightly different proteins to be derived from the same gene.
- Such modifications enable the cells to generate numerous proteins from a limited DNA set / better regulation at gene level.
- As an instance, alternative splicing of pre-mRNA for α-tropomyosin results in different protein isoforms in muscle and non-muscle tissue.
- Also, modifications serve as mRNA for export – the nuclear pores are only allowing the molecules that are properly capped and spliced to pass into the cytoplasm.
FAQ
What is post-transcriptional modification?
Post-transcriptional modification refers to the chemical modifications that occur to RNA molecules after they have been transcribed from DNA. These modifications can impact RNA stability, localization, and functionality.
What are some common types of post-transcriptional modifications?
Common types of PTMs include alternative splicing, RNA editing, polyadenylation, capping, methylation, and various forms of non-coding RNA modifications.
How does alternative splicing contribute to PTM?
Alternative splicing is a PTM process where different exons within a pre-mRNA can be selectively included or excluded, resulting in the production of multiple protein isoforms from a single gene.
What is the role of RNA editing in post-transcriptional modification?
RNA editing involves the alteration of specific nucleotides within an RNA molecule, leading to changes in the RNA sequence. This modification can impact protein coding potential, RNA structure, and stability.
How does polyadenylation affect RNA molecules?
Polyadenylation involves the addition of a string of adenine nucleotides (poly-A tail) to the 3′ end of an RNA molecule. This modification can influence RNA stability, translation efficiency, and nuclear export.
What is the significance of RNA methylation?
RNA methylation involves the addition of a methyl group to RNA nucleotides. It plays a role in regulating RNA stability, splicing, translation, and interactions with other RNA-binding proteins.
How does capping affect mRNA?
Capping refers to the addition of a modified guanosine nucleotide (m7G cap) to the 5′ end of an mRNA molecule. This modification protects the mRNA from degradation, facilitates mRNA export from the nucleus, and enhances translation efficiency.
What are the functions of non-coding RNA modifications?
Non-coding RNAs, such as transfer RNA (tRNA) and ribosomal RNA (rRNA), undergo various modifications that can impact their structure, stability, and functions in translation and protein synthesis.
Are post-transcriptional modifications reversible?
Yes, some PTMs can be reversible. For example, RNA methylation can be dynamically added or removed, allowing for rapid regulation of RNA function in response to cellular signals or environmental changes.
How do post-transcriptional modifications contribute to gene expression regulation?
PTMs play a crucial role in fine-tuning gene expression. They can affect RNA stability, splicing patterns, translation efficiency, and interactions with regulatory proteins, ultimately influencing the abundance and functionality of proteins in the cell.
- Alberts, B., Johnson, A., Lewis, J., et al. (2002). Molecular Biology of the Cell (4th ed.). Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK26890/
- Wong, E. V. (n.d.). Post Transcriptional Modifications. Axolotl Academica Publishing. http://www.axopub.com/wp01/category/cells/
- Cold Spring Harbor Laboratory Press. (2017). Molecular Biology of the Gene (7th ed.). Cold Spring Harbor, New York.
- Lewin, B. (2008). GENE IX. Jones and Bartlett Publishers.
- Verma, P. S., & Agarwal, V. K. (2005). Cell Biology, Genetics, Molecular Biology, Evolution and Ecology. S. Chand & Company Ltd.
- Principles of Genetics, D. Peter Snustad, Michael J. Simmons.
- Biochemistry, Dr. U. Satyanarayana, Dr. U. Chakrapani.
- https://en.wikipedia.org/wiki/Post-transcriptional_modification
- https://www.basu.org.in/wp-content/uploads/2021/06/Post-Transcriptional-Modifications_1.pdf
- https://www.slideshare.net/sadiqpa/post-transcriptional-modification
- https://www.slideshare.net/AYSHA007/posttranslational-modifications
- https://www.slideshare.net/saadiaeman/post20translational20modifications
- https://www.slideshare.net/sujay45/posttranslational-modification
- https://plantlet.org/post-transcriptional-modification/
- https://en.wikipedia.org/wiki/Alternative_splicing