What is Plasmodium Vivax?
- Plasmodium vivax is a protozoan parasite that primarily infects humans and is a significant causative agent of malaria. This organism is noteworthy for being the most prevalent species responsible for recurring malaria infections worldwide. While it is not as lethal as Plasmodium falciparum—the most dangerous of the five known human malaria parasites—P. vivax can still result in severe illness and even death. Such outcomes are often attributed to complications like splenomegaly, a condition characterized by an abnormal enlargement of the spleen.
- P. vivax is transmitted through the bite of infected female Anopheles mosquitoes, as males do not engage in biting. The life cycle of this parasite is complex, involving two distinct hosts: the female Anopheles mosquito, which serves as the definitive or primary host, and humans, who act as the intermediate or secondary host. This digenetic life cycle consists of both sexual and asexual reproduction phases.
- In the mosquito, P. vivax undergoes a sexual phase known as gametogony, followed by sporogony. During gametogony, male and female gametes are formed, which subsequently fuse to create zygotes. These zygotes develop into sporozoites, which migrate to the mosquito’s salivary glands. When the mosquito bites a human, these sporozoites are injected into the bloodstream, initiating infection.
- Once inside the human host, P. vivax predominantly resides within red blood cells and liver cells. This stage of the life cycle is referred to as the asexual phase, characterized by schizogony. During schizogony, the parasite multiplies within the host’s cells, eventually causing the red blood cells to rupture, which leads to the clinical symptoms associated with malaria, such as fever and chills.
- One unique aspect of P. vivax is its ability to remain dormant in the liver in a form known as hypnozoites. This dormant stage can reactivate, leading to relapses of malaria symptoms even after successful treatment. Consequently, the management of P. vivax malaria requires specific antimalarial medications, such as primaquine, to eliminate these dormant forms and prevent recurrences.
Epidemiology of Plasmodium Vivax
The epidemiology of Plasmodium vivax, a significant human malaria parasite, reveals crucial insights into its global distribution, risk factors, and unique characteristics. Understanding these aspects is essential for developing effective control strategies and mitigating the burden of this disease.
- P. vivax is the most widespread species among malaria-causing parasites, with more than one-third of the global population—approximately 2.5 billion individuals—at risk of infection.
- This species can thrive in a broader range of climates, including temperate zones, due to its dormant liver stage, allowing it to persist even in colder environments.
- The highest prevalence of P. vivax infections occurs in Latin America and Southeast Asia, with significant public health implications in these regions.
- The World Health Organization’s 2018 World Malaria Report indicated that, in 2017, 74.1% of malaria cases in the Region of the Americas were attributed to P. vivax.
- In several Southeast Asian countries, such as South Korea, P. vivax is the sole species responsible for malaria infections, showcasing its geographic adaptability.
- A unique feature of P. vivax is its dependence on the Duffy antigen for entering red blood cells. Consequently, its prevalence is notably lower in Africa, where a significant portion of the population lacks this antigen.
- Traditional beliefs suggesting that the absence of the Duffy antigen provides complete immunity against P. vivax infections have been challenged by recent findings showing sporadic cases in Duffy-null African populations.
- Immunity acquisition against P. vivax occurs more rapidly compared to P. falciparum. Thus, in endemic regions, the morbidity associated with P. vivax peaks at a younger age, and adults tend to experience asymptomatic infections more frequently.
- In contrast, in areas with low transmission rates, the risk of severe disease is not age-dependent, highlighting the complexity of P. vivax infections across different settings.
In the United States, malaria remains a public health concern, although cases are relatively rare.
- The Centers for Disease Control and Prevention (CDC) reports approximately 1,700 malaria cases diagnosed annually in the U.S., with a notable portion attributed to P. vivax.
- A retrospective study conducted by Newman et al. (2004) revealed that only 3.3% of malaria-related deaths between 1963 and 2001 were due to P. vivax, compared to 92.7% for P. falciparum.
- The study emphasized that most malaria deaths are preventable, often resulting from factors such as non-adherence to chemoprophylaxis, improper prophylactic measures, and delayed diagnosis.
Geographic Distribution of Plasmodium Vivax
The geographic distribution of Plasmodium vivax is influenced by environmental conditions conducive to malaria transmission. Understanding the patterns of distribution is critical for addressing malaria control and prevention strategies.
- Current Distribution:
- P. vivax predominantly exists in tropical and subtropical regions, where climatic conditions support the life cycle of the malaria vector, primarily the Anopheles mosquito.
- This species is typically found at altitudes below 1,500 meters, as higher elevations tend to be less hospitable for the mosquito vectors.
- Historical Context:
- Historically, malaria, including infections caused by P. vivax, was endemic across large portions of North America, Europe, and northern Asia.
- While the prevalence of malaria has significantly declined in these regions, traces of P. vivax remain, particularly in the Korean peninsula.
- Complementary Niches with Other Species:
- In terms of ecological niches, P. vivax and P. ovale are traditionally viewed as occupying complementary environments; P. ovale is more common in Sub-Saharan Africa, while P. vivax is prevalent in other areas.
- However, their geographical distributions overlap, and thus, understanding their exact ranges is complex.
- Morphological and Molecular Distinctions:
- Distinguishing between P. vivax and P. ovale based solely on morphological characteristics can be challenging.
- The application of molecular diagnostic tools is essential for accurately identifying these species and determining their specific geographic distributions.
- Global Distribution of Other Species:
- While P. falciparum is the most prevalent and significant species responsible for malaria worldwide, P. malariae also has a broad distribution in South America, Asia, and Africa, although it is less frequently associated with malaria cases compared to P. falciparum.
- P. knowlesi, another species of Plasmodium, is found primarily in Southeast Asia and has been gaining attention due to its zoonotic transmission pathways.
- Impact of Climatic Changes and Population Movements:
- The current distribution of P. vivax and other malaria species is subject to change due to various factors such as climatic shifts and human population movements.
- These changes can alter the ecological balance and facilitate the spread of malaria into new regions where it was previously absent.
Classification of Plasmodium vivax
The classification of Plasmodium vivax is essential for understanding its biological characteristics and ecological relationships. This protozoan parasite is categorized within various taxonomic levels that reflect its evolutionary history and functional attributes.
- Domain: Plasmodium vivax belongs to the domain Eukaryota. This classification indicates that it possesses a complex cell structure, including a nucleus and other membrane-bound organelles.
- Unranked Taxa:
- Diaphoretickes: This unranked group includes diverse eukaryotic organisms that share certain evolutionary traits.
- Clade TSAR: This clade is a higher-level grouping within the SAR supergroup, representing a diverse array of protists.
- Clade SAR: This clade encompasses a large variety of organisms, including several important groups of protists and other eukaryotic life forms.
- Infrakingdom: P. vivax is classified under the infrakingdom Alveolata. This group is characterized by the presence of membrane-bound sacs called alveoli, which play a role in various cellular functions.
- Phylum: The parasite is placed within the phylum Apicomplexa. Members of this phylum are predominantly intracellular parasites and are known for their complex life cycles and unique organelles, such as the apical complex, which aids in host cell invasion.
- Class: Within the Apicomplexa, P. vivax is further classified in the class Aconoidasida. This class comprises organisms that lack certain structures typically found in other Apicomplexan classes, specifically the apical complex.
- Order: P. vivax belongs to the order Haemospororida. This order includes various genera and species that primarily infect vertebrates and are transmitted by arthropod vectors.
- Family: The family Plasmodiidae encompasses several genera, including Plasmodium, which are responsible for causing malaria. This family is characterized by its members’ parasitic nature and their reliance on both mosquito vectors and vertebrate hosts.
- Genus: Within the family Plasmodiidae, P. vivax is classified under the genus Plasmodium. This genus is known for its species that infect humans and other vertebrates, leading to malaria.
- Species: Finally, the specific epithet is vivax, distinguishing this particular species from others within the genus Plasmodium. P. vivax is notable for causing relapsing malaria, due to its ability to form dormant liver stages.
Discovery
The discovery of Plasmodium vivax is a significant milestone in the study of malaria and protozoan parasites. Its identification marked an important advancement in understanding the complexities of malaria infections in humans.
- Historical Context: The genus Plasmodium was first described in 1885 by Italian scientists Ettore Marchiafava and Angelo Celli. This discovery laid the groundwork for further research into various species within this genus, which now includes over 200 identified species.
- Classification: P. vivax is among the 11 species known to infect humans, contributing to the global burden of malaria. It is classified as a malaria parasite due to its role in causing malaria.
- Diversity of Hosts: In addition to its human hosts, P. vivax is part of a larger group of Plasmodium species that infect other animals, including primates, rodents, birds, and reptiles. This indicates a wide range of ecological interactions and potential reservoirs for transmission.
- Life Cycle: The life cycle of P. vivax involves two essential hosts: a vector, typically an Anopheles mosquito, and a vertebrate host, such as humans. This digenetic life cycle underscores the importance of understanding both the vector and host dynamics in controlling malaria transmission.
- Species Overview: Among the four main species responsible for human malaria, P. vivax is recognized alongside Plasmodium falciparum, Plasmodium malariae, and Plasmodium ovale. While P. falciparum is noted for its high mortality rates, P. vivax is also significant due to its ability to cause relapsing malaria, attributed to its dormant liver stage.
- Impact on Public Health: The identification and characterization of P. vivax have had profound implications for public health strategies aimed at malaria control and treatment. Understanding its unique life cycle, including the ability to remain dormant and reactivate, is crucial for developing effective interventions.
- Current Research: Ongoing studies focus on the genetic and biological aspects of P. vivax to develop more effective diagnostic tools, treatments, and preventive measures. This research is essential for addressing the challenges posed by this parasite in endemic regions.
Characteristics of Plasmodium Vivax
- Morphology: Plasmodium vivax is a eukaryotic, unicellular parasite that belongs to the class Apicomplexa. Its life cycle is comprised of numerous phases in both the human host and the mosquito vector.
- Size: The parasite’s diameter ranges from 1 to 4 microns, which is smaller than a red blood cell.
- Genome: P. vivax’s genome is roughly 48 megabases in size and contains around 5,600 predicted genes. Its genetic variety adds to its capacity to escape the human immune system.
- Transmission: P. vivax is transmitted to humans by female Anopheles mosquitoes that are infected with the parasite. P. vivax, unlike certain other Plasmodium species, can be transmitted by both daytime and nighttime mosquito bites.
- Life Cycle: P. vivax’s life cycle consists of several phases, including sporozoites, merozoites, and gametocytes. After being transmitted by an infected mosquito to a human host, the sporozoites migrate to the liver, where they infect hepatocytes and multiply to generate hundreds of merozoites. These merozoites enter the bloodstream and infect red blood cells, resulting in malaria symptoms. Certain merozoites transform into gametocytes, which can be ingested by a mosquito during a blood meal to conclude the sexual phase of the parasite’s life cycle.
- Hypnozoites: P. vivax is capable of forming hypnozoites, which are dormant liver stages that can persist in the liver for months or even years before reactivating and causing malaria relapses.
- Drug Resistance: P. vivax has developed resistance to certain antimalarial medications, especially in Southeast Asia. This has complicated the treatment of P. vivax malaria, as effective medications must be able to target both the parasite’s blood and liver stages.
- Geographical Distribution: Plasmodium vivax is the most globally dispersed of all malaria parasites, having the highest incidence in Asia and the Pacific. It is also widespread in portions of Africa, Latin America, and the Middle East.
- Symptoms: P. vivax malaria shares symptoms with other kinds of malaria, including fever, chills, headache, muscle pains, and weariness. Typically, symptoms occur 10 to 15 days following infection.
- Treatment: Treatment options for P. vivax include antimalarial medicines such as chloroquine, primaquine, and artemisinin-based combination treatments. However, the existence of hypnozoites, which can trigger relapses of the illness, can complicate treatment.
- Prevention: The most effective method for preventing P. vivax malaria is to avoid mosquito bites by using insect repellent, wearing protective clothes, and sleeping under a mosquito net. Additional preventative strategies include eliminating standing water where mosquitoes grow and taking antimalarial medication before travelling to malaria-endemic regions.
- Impact: Malaria caused by P. vivax is a severe threat to public health, producing significant morbidity and mortality, especially among children under the age of 5. In addition, it has a huge impact on the economy due to lost production and medical expenses. The presence of hypnozoites, which can trigger relapses of the infection months or even years after the initial infection, has hampered control and eradication attempts.
- Vector: Plasmodium vivax is transmitted to humans predominantly through the bite of female Anopheles mosquitoes, which serve as the parasite’s vector. These mosquitoes are mainly found in tropical and subtropical regions of the world and bite between dusk and dawn.
- Host: Plasmodium vivax infects humans and other primates, such as macaques and chimpanzees. The principal reservoir for the parasite is the human host, and transmission occurs when an infected mosquito feeds on human blood and injects sporozoites into the bloodstream.
- Incubation Time: Plasmodium vivax normally incubates between 10 and 15 days after the bite of an infected mosquito. This indicates that the infection’s symptoms, including fever, chills, headache, muscle pains, and weariness, typically manifest within this timeframe. However, in rare instances, the incubation period might be longer or shorter depending on factors such as the individual’s immunological condition, the number of transmitted parasites, and the parasite strain. It is also crucial to note that P. vivax is capable of producing hypnozoites, which can linger in the liver for months or even years before reactivating and causing malaria relapses. This can complicate the identification and treatment of P. vivax infections and highlights the significance of recurrence surveillance after therapy.
Host of Plasmodium vivax
The host dynamics of Plasmodium vivax are crucial for its life cycle and transmission. Understanding the roles of the human body and the female Anopheles mosquito as hosts provides insight into the mechanisms of malaria spread.
- Primary Host: The human body serves as the primary host for P. vivax. Within this host, the parasite undergoes asexual reproduction, which is essential for its development and propagation. The infection primarily targets red blood cells and liver cells, where the parasite multiplies and causes the clinical symptoms associated with malaria.
- Secondary Host: The female Anopheles mosquito acts as the secondary or intermediate host. This stage of the life cycle is critical for the sexual reproduction of P. vivax. The mosquito becomes infected when it feeds on the blood of an infected human, acquiring gametocytes from the blood.
- Transmission Process: Upon ingestion of the human blood containing gametocytes, the parasite undergoes gametogony in the mosquito’s digestive system. Male and female gametes fuse to form zygotes, which then develop into sporozoites. These sporozoites migrate to the mosquito’s salivary glands, enabling transmission to a new human host during subsequent feeding.
- Vector Species: Various species of Anopheles mosquitoes facilitate the transmission of P. vivax malaria. Notable vectors include:
- Anopheles maculatus: Common in certain regions, this species is recognized for its role in malaria transmission.
- Anopheles stephensi: This species is particularly significant in urban areas, contributing to the spread of malaria in densely populated regions.
- Anopheles fluviatilis: Found in specific geographical areas, it serves as another important vector for the parasite.
- Anopheles culicifacies: This mosquito species is also involved in transmitting P. vivax and plays a role in malaria epidemiology.
- Ecological Interactions: The interaction between the human host and the Anopheles mosquito is vital for the survival of P. vivax. The parasite relies on both hosts to complete its life cycle, demonstrating a complex relationship that facilitates transmission and persistence within populations.
- Public Health Implications: Understanding the host dynamics of P. vivax is essential for developing effective malaria control strategies. Targeting both the human and mosquito populations can significantly reduce transmission rates and the incidence of malaria.
Various forms in the life cycle of Plasmodium
- Sporozoite – The sporozoite of Plasmodium vivax is elongated and around 15 µm in length. The virus is kept in the salivary glands of mosquitoes and is transmitted to humans. They contain thick pellicles and fibres on their periphery that aid in movement.
- Schizont — The adult liver schizont has a diameter of 40-80 µm. Upon rupture, merozoites are released. Erythrocytic schizonts are 7-8 µm in length and are the same size as RBCs. They have several nuclei and multiple merozoites.
- Immature trophozoites – The Plasmodium vivax immature trophozoites are ring-shaped and are formed in the erythrocytes. About 1-2 µm in diameter.
- Mature Plasmodium trophozoites – The form of mature Plasmodium trophozoites is uneven (amoeboid). They include haemozoin, a dark pigment generated from the haemoglobin of the infected RBCs.
- Gametocytes -The gametocytes range in size from 7 to 14 µm. The form and size of gametocytes vary amongst Plasmodium species. In the RBCs, gametocytes are generated.
- Zygote – Resulting from the fusion of micro and mega gametocytes.
- Ookinete – The zygote transforms into mobile ookinetes. They are around 15-20 µm in length and are elongated. They move through the mosquito’s midgut wall and transform into oocysts.
- Oocyst – It has a diameter of around 50 µm. The oocysts transform into sporozoites that enter and are retained in the salivary glands.
Habitat of Plasmodium vivax
The habitat of Plasmodium vivax is crucial for its survival, transmission, and proliferation. This protozoan parasite relies on specific environmental conditions and hosts, predominantly in tropical and subtropical regions.
- Geographical Distribution: P. vivax is primarily found in hotspots across Asia, Latin America, and parts of Africa. These regions provide the warm, humid conditions favorable for the development of both the parasite and its mosquito vectors.
- Origins: Research indicates that P. vivax may have originated from wild chimpanzees and gorillas in central Africa. This zoonotic potential underscores the complex evolutionary history of the parasite and its adaptation to various hosts.
- Epidemiology: The protozoan is responsible for approximately 65% of malaria cases in Asia and South America, highlighting its significant impact on public health in these regions. The widespread prevalence of P. vivax necessitates ongoing surveillance and control measures.
- Temperature Influence: P. vivax can undergo sporogonic development at lower temperatures, similar to Plasmodium falciparum. This adaptability allows the parasite to thrive in a range of environmental conditions, which is essential for its survival in diverse habitats.
- Mosquito Species: There are around 71 species of mosquitoes that can serve as vectors for P. vivax. This extensive diversity of mosquito hosts facilitates the transmission of the parasite, as different species may inhabit varying ecological niches.
- Preferred Habitats: The ideal habitats for P. vivax include:
- Tropical and Subtropical Regions: Warm climates with ample rainfall support the breeding of Anopheles mosquitoes, which are essential for the parasite’s transmission.
- Stagnant Water Sources: Mosquitoes typically breed in stagnant or slow-moving water, such as ponds, marshes, and rice fields, creating suitable environments for both the vector and the parasite.
- Human Habitats: Human settlements in rural and semi-urban areas often coincide with mosquito breeding sites, increasing the likelihood of transmission. Therefore, understanding human behavior and environmental factors is critical for malaria control.
Morphology of Plasmodium vivax
The structure of Plasmodium vivax is complex and adapted to its role as an intracellular parasite. A thorough understanding of its ultrastructure is vital for comprehending its life cycle, pathogenesis, and interactions with host cells.
- Ultrastructural Observations: Detailed observations of P. vivax have been conducted using electron microscopy. This advanced imaging technique allows researchers to visualize the intricate details of the parasite’s cellular components.
- Trophozoite Stage: The trophozoite, which is the feeding and growing stage of the parasite, exhibits characteristic features when stained with Giemsa. The presence of Schüffner’s dots is notable, which are small, stippled inclusions in the cytoplasm that play a role in identifying the species.
- Cellular Membranes: Inside the red blood cell, P. vivax is enclosed by a double membrane. The plasmalemma, or the outermost membrane, is closely apposed to the cytoplasm, facilitating nutrient absorption and interaction with the host cell.
- Cytoplasm Composition: The cytoplasm of P. vivax contains ribonucleoproteins, which are complexes of RNA and proteins that are essential for various cellular functions. It also consists of small, dense particles critical for the parasite’s metabolism.
- Endoplasmic Reticulum: The endoplasmic reticulum (ER) in P. vivax resembles vesicles of variable shapes, which are not well-developed compared to other eukaryotic organisms. These ER vesicles can be either smooth or rough-surfaced, depending on their function, and they remain scattered throughout the cytoplasm.
- Mitochondrial Structure: Mitochondria in P. vivax are surrounded by a double membrane that features peripheral cristae, which are folds that increase the surface area for metabolic processes. The central region of the mitochondria is structureless. The number of mitochondria varies with the developmental stage; for example, merozoites contain a single mitochondrion, while trophozoites possess multiple mitochondria.
- Golgi Apparatus: The Golgi apparatus is composed of several small vesicles arranged in rows, which are involved in processing and transporting proteins and lipids within the parasite.
- Concentric Bodies: Attached to the plasmalemma is a double-layered concentric body. Rudzinska proposed in 1965 that these structures might serve essential mitochondrial functions, potentially compensating for the unique adaptations of the parasite.
- Vacuoles: The cytoplasm also contains one or two double-membrane vacuoles filled with a structureless matrix. Although the specific functions of these vacuoles remain unclear, they may play roles in storage or metabolic processes.
- Nucleus Structure: P. vivax has a larger nucleus compared to many other cells. The nucleoplasm is composed of granular and fine fibrillar material, and the nucleus is encased in a double membrane with RNA particles attached. The centrally located nucleolus is involved in ribosome synthesis.
- Pinocytosis Vacuoles: Pinocytosis vacuoles function as food vacuoles, engulfing nutrients from the host. These vacuoles can sometimes contain hemozoin, a byproduct of hemoglobin digestion, which varies depending on the species of Plasmodium.
Genome Structure of Plasmodium vivax
The genome structure of Plasmodium vivax plays a critical role in its biology and pathogenicity. Understanding the organization and composition of its genetic material is essential for grasping how this parasite functions and evolves.
- Chromosomal Structure: P. vivax has a total of 12 to 14 linear chromosomes. This linear arrangement is characteristic of many eukaryotic organisms, and it differentiates P. vivax from other malaria parasites that may exhibit different chromosomal configurations.
- Genome Size: The estimated genome size of P. vivax ranges from 35 to 40 megabases (Mb). This size reflects the complexity of the organism and provides insight into the number and types of genes it encodes.
- Isochores: The genome exhibits GC-rich isochores, which are regions with a higher guanine-cytosine content. These GC-rich areas are typically associated with protein-coding genes, indicating a higher density of functional genes in these regions. Conversely, AT-rich isochores are found at the chromosome ends, or telomeres. This structural distinction may play a role in chromosome stability and gene expression regulation.
- Gene Count: It is estimated that the P. vivax genome contains approximately 5,000 genes. These genes are responsible for encoding proteins that facilitate various cellular processes, including metabolism, replication, and interaction with the host.
- Functional Implications: The specific arrangement of genes and the overall structure of the genome are crucial for the parasite’s ability to adapt to different hosts and environments. For instance, genes involved in evading the host immune response, as well as those required for the parasite’s development in mosquitoes and humans, are vital for its survival.
Life Cycle of Plasmodium vivax
The life cycle of Plasmodium vivax, a protozoan parasite responsible for malaria, is a complex process that involves both asexual and sexual reproduction. This life cycle occurs in two hosts: humans and female Anopheles mosquitoes. Understanding the sequential stages is crucial for developing effective malaria control strategies.
- Overview of Life Cycle Stages:
The life cycle of P. vivax consists of distinct phases that take place in humans (asexual reproduction) and in mosquitoes (sexual reproduction).
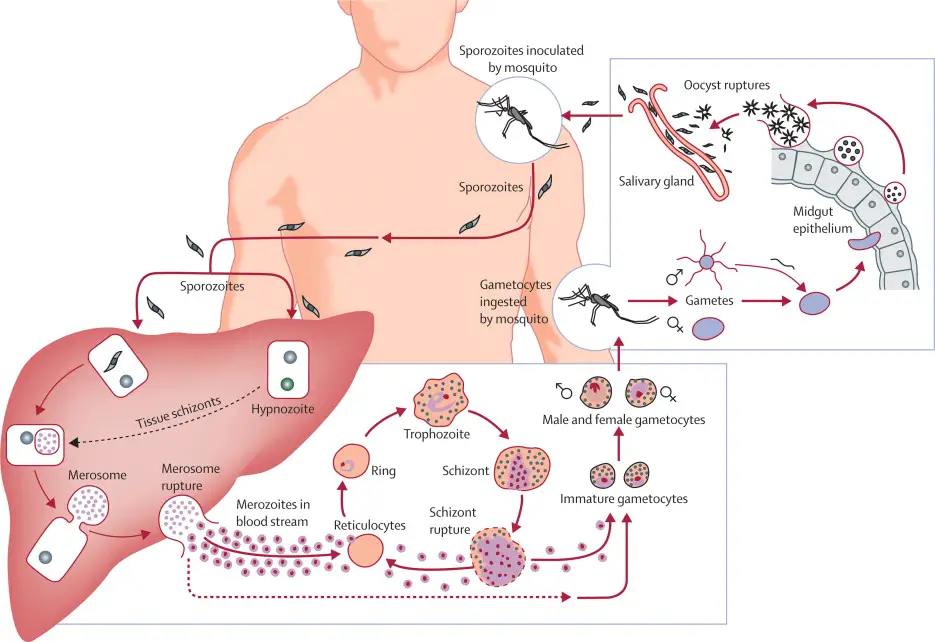
Life Cycle in Humans (Asexual Cycle)
- Inoculation:
- During a blood meal, an infected female Anopheles mosquito injects sporozoites into the human bloodstream.
- These sporozoites measure 11 to 12 microns in length and 0.5 to 1 micron in width, displaying a sickle shape with an oval nucleus.
- They move through the bloodstream and migrate to the liver.
- Schizogony in Liver Cells:
- Once in the liver, sporozoites develop into large, round schizont cells.
- The schizont undergoes multiple fissions, resulting in thousands of spindle-shaped cells known as merozoites, through a process called schizogony.
- This phase of asexual multiplication is known as pre-erythrocytic schizogony, and the produced merozoites are termed cryptozoites or cyptomerozoites.
- These cryptozoites are resistant to medications and immune responses from the host.
- Exo-Erythrocytic Schizogony:
- A further stage of asexual reproduction occurs as cryptozoites enter new liver cells, developing into new schizonts.
- These second-generation merozoites are known as metacryptozoites (or phanerozoites).
- Metacryptozoites can be categorized into micro-metacryptozoites, which infect red blood cells, and macro-metacryptozoites, which continue developing in liver cells.
- Pre-Patent and Incubation Periods:
- The pre-patent period, which spans from sporozoite infection to the detection of parasites in the blood, averages 8 days.
- The incubation period, the time from sporozoite infection until the onset of malaria symptoms, typically ranges from 10 to 17 days.
- Schizogony in Erythrocytes:
- In this phase, a ring-shaped trophozoite forms when a micro-metacryptozoite invades an erythrocyte, leading to the formation of a vacuole and displacing the nucleus to one side.
- This initial stage is referred to as the signet ring stage and measures one-third to one-half the size of the erythrocyte.
- The trophozoite develops into a rounded schizont, which then undergoes another round of schizogony, producing 12 to 24 oval-shaped merozoites.
- Upon bursting of the erythrocyte, these merozoites are released into the plasma, where they can invade new erythrocytes, continuing the cycle approximately every 48 hours.
- Formation of Gametocytes:
- Merozoites develop into two types of gametocytes: macrogametocytes (female) and microgametocytes (male).
- The precise mechanisms behind gametocyte formation are not fully understood.
- Macrogametocytes are characterized by food-rich cytoplasm and a small eccentric nucleus, while microgametocytes feature clear cytoplasm and a large central nucleus.
- Both types contain hemozoin and lead to enlargement of the erythrocytes. Gametocytes are then transmitted to the mosquito during a blood meal.
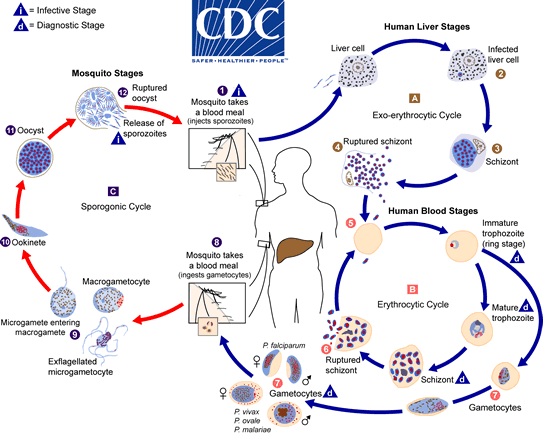
Life Cycle in Mosquito (Sexual Cycle)
- Gametogony:
- Upon entering the mosquito’s stomach, microgametocytes undergo ex-flagellation, producing 6-8 haploid daughter nuclei.
- These nuclei migrate to the periphery of the microgametocyte, and the cytoplasm forms flagellum-like structures, resulting in the formation of microgametes, which are 20-25 microns long.
- Fertilization:
- Syngamy occurs when a microgamete fertilizes a macrogamete, resulting in the formation of a zygote.
- This fusion is termed anisogamy due to the dissimilar sizes of the gametes.
- Ookinete Formation and Encystment:
- After approximately 24 hours, the zygote elongates and becomes motile, transforming into an ookinete (15-22 microns in length).
- The ookinete penetrates the stomach wall of the mosquito and lies beneath the epithelial layer, developing into an oocyst surrounded by a thin, elastic membrane.
- Sporogony:
- Within the oocyst, meiotic and mitotic divisions occur, resulting in the formation of numerous haploid sporozoites.
- A single oocyst can generate up to 10,000 sporozoites through sporogony.
- When the oocyst ruptures, the sporozoites are released into the mosquito’s hemolymph, migrating to the salivary glands.
- Transmission to Humans:
- During a subsequent blood meal, the infected mosquito injects sporozoites into the bloodstream of a human, thus continuing the life cycle of P. vivax.
Pathogenicity of Plasmodium Vivax
- Despite its low virulence, Plasmodium vivax is now recognised as one of the leading causes of severe and deadly malaria. Blood cells infected with vivax become malformed despite the apparent rarity of parasite sequestration.
- Severe amenorrhea is observed alongside repeated hemolysis of the majority of healthy erythrocytes with heightened susceptibility. Inflammation of the alveolar-capillary membrane’s permeability causes lung damage. Nevertheless, vivax-related coma is uncommon.
- A double membrane is a plasmodium characteristic of the red blood cell, and the plasmalemma is intimately attached to the cytoplasm. Plasmodium vivax’s cytoplasm is composed of ribonucleoproteins comprising small, dense particles.
- Mitochondria carrying the malaria parasite Plasmodium vivax have a twofold membrane with peripheral cristae and a more rigid, less central area.
- The life cycle of Plasmodium vivax is separated into two stages: asexual life or schizogony in males and sexual life cycle in female Anopheles mosquitoes.
- In schizogony, Plasmodium vivax in humans and plasmodium reproduce asexually in liver and RBC cells. An infected female Anopheles mosquito with sporozoites in its salivary gland infects a man.
- As it punctures the man’s skin, the mosquito injects its infected saliva into the man’s circulation. The sporozoites multiply by the hundreds in the blood of the host. Sporozoites are the parasite’s inflected forms.
Diagnosis of Plasmodium Vivax disease (malaria)
The diagnosis of Plasmodium vivax malaria involves a systematic approach utilizing various methods to identify the presence of the parasite. Accurate diagnosis is essential for effective treatment and management of the disease. Below are the key diagnostic modalities:
- Microscopic Examination:
- Microscopy remains the definitive “gold standard” for diagnosing malaria. The examination involves analyzing thick and thin blood smears stained with Giemsa, Wright, or Wright-Giemsa stains.
- Giemsa Staining: This method is preferred due to its ability to highlight distinctive morphological features of the parasite, such as Schüffner’s dots and Maurer’s clefts, which may not be visible with other stains.
- Thick Smears: These are used primarily to detect the presence of parasites, as they concentrate a larger volume of blood, increasing the likelihood of detection.
- Thin Smears: These are utilized for species-level identification and quantification of parasitemia.
- Morphological Features: The stages of P. vivax observed in blood smears include:
- Ring Stage: Normal to 1.25x size; characterized by occasional fine Schüffner’s dots and large cytoplasm with pseudopods.
- Trophozoite Stage: Enlarged (1.5 to 2x), exhibiting amoeboid cytoplasm and fine yellowish-brown pigment.
- Schizont Stage: Enlarged (1.5 to 2x), with multiple merozoites and yellowish-brown pigment.
- Gametocyte Stage: Enlarged (1.5 to 2x), either round or oval, with compact chromatin and scattered brown pigment.
- Molecular Diagnosis:
- Molecular methods enhance the accuracy of diagnosis, particularly in cases where microscopy may yield inconclusive results due to overlapping morphologies.
- Polymerase Chain Reaction (PCR): This technique allows for species-specific detection of Plasmodium DNA. The procedure includes:
- Extraction of genomic DNA from blood samples.
- Use of a dual duplex real-time PCR assay that distinguishes between P. falciparum, P. vivax, P. malariae, and P. ovale.
- In cases of suspected mixed infections, a conventional nested PCR can be employed for improved resolution.
- Antibody Detection:
- The Indirect Fluorescent Antibody (IFA) test detects antibodies against Plasmodium antigens in patient serum. Although not suitable for acute malaria diagnosis due to the delayed antibody response, this test can be valuable for:
- Screening blood donors to prevent transfusion-induced malaria.
- Evaluating patients post-treatment to confirm past infections.
- The IFA uses schizont antigens and fluorescein-labeled antihuman antibodies to visualize positive reactions under fluorescence microscopy, with successful binding producing a characteristic apple green fluorescence.
- The Indirect Fluorescent Antibody (IFA) test detects antibodies against Plasmodium antigens in patient serum. Although not suitable for acute malaria diagnosis due to the delayed antibody response, this test can be valuable for:
- Antigen Detection:
- Rapid Diagnostic Tests (RDTs) are employed for the quick detection of malaria antigens in clinical settings. These tests are user-friendly and do not require sophisticated laboratory equipment.
- Common antigen targets in these tests include:
- Histidine Rich Protein-2 (HRP-2): Associated specifically with P. falciparum.
- Plasmodium-specific Aldolase: Another marker for detection.
- Plasmodium-associated Lactate Dehydrogenase (pLDH): Can be identified through enzymatic activity or immunoassay techniques.
Treatment of Plasmodium Vivax disease (malaria)
The treatment of Plasmodium vivax malaria necessitates a multifaceted approach, primarily guided by the sensitivity of the parasite to specific antimalarial medications. Understanding the treatment regimen is essential for effectively managing and curing the disease.
- First-Line Treatment:
- For uncomplicated P. vivax malaria, Chloroquine remains the principal therapeutic agent in regions where the parasite is chloroquine-sensitive.
- Treatment involves:
- Chloroquine Phosphate:
- Initial dose: 600 mg base (1,000 mg salt) orally.
- Follow-up doses: 300 mg base (500 mg salt) orally at 6, 24, and 48 hours.
- Total Dose: 1,500 mg base (2,500 mg salt).
- Hydroxychloroquine:
- Initial dose: 620 mg base (800 mg salt) orally.
- Follow-up doses: 310 mg base (400 mg salt) orally at 6, 24, and 48 hours.
- Chloroquine Phosphate:
- Combination Therapy:
- To eliminate the dormant liver stage of the parasite, it is crucial to include Primaquine in the treatment regimen.
- Primaquine Phosphate:
- Dosage: 30 mg base orally once daily for 14 days.
- Resistance Considerations:
- In regions where chloroquine resistance is prevalent, particularly in Papua New Guinea and Indonesia, alternative treatment options are necessary. The Centers for Disease Control and Prevention (CDC) recommends three options:
- Atovaquone-Proguanil
- Quinine combined with either Tetracycline or Doxycycline
- Mefloquine
- Any of these alternatives should be paired with Primaquine to address the hypnozoite phase.
- In regions where chloroquine resistance is prevalent, particularly in Papua New Guinea and Indonesia, alternative treatment options are necessary. The Centers for Disease Control and Prevention (CDC) recommends three options:
- Contraindications for Primaquine:
- G6PD Deficiency:
- Patients with G6PD deficiency are at risk for severe hemolytic anemia when treated with Primaquine. Its use must be carefully evaluated on a case-by-case basis.
- Pregnancy:
- Due to the potential for fetal hemolysis, Primaquine is contraindicated during pregnancy. Treatment should be postponed until after delivery.
- G6PD Deficiency:
- Severe Malaria Treatment:
- The World Health Organization (WHO) recommends Intravenous Artesunate for severe malaria, which is administered as follows:
- Dosage: 2.4 mg/kg IV or IM at 0, 12, and 24 hours, followed by daily doses.
- Artesunate has shown superior efficacy and safety compared to intravenous quinidine, which has traditionally been used.
- Quinidine is also recommended by the CDC for treating severe malaria in the United States, with the requirement for continuous cardiac monitoring due to the risk of arrhythmias associated with its administration.
- The World Health Organization (WHO) recommends Intravenous Artesunate for severe malaria, which is administered as follows:
- Investigational Use:
- Intravenous Artesunate is accessible in the United States through an investigational new drug protocol for cases of severe malaria that do not respond to alternative treatments or when intravenous quinidine is unavailable.
FAQ
What is the habitat of Plasmodium vivax?
Plasmodium vivax is a parasite that causes malaria in humans. It is primarily found in tropical and subtropical regions, where the Anopheles mosquito, which transmits the parasite, thrives. The habitat of Plasmodium vivax includes areas with high humidity and rainfall, such as forests, marshes, and areas near stagnant water bodies. These conditions are ideal for the breeding and survival of Anopheles mosquitoes, which require water for their larval development. Plasmodium vivax can also survive in temperate regions, where the climate is suitable for the Anopheles mosquito to breed. It is important to note that the habitat of Plasmodium vivax is not limited to any specific geographical location, and the parasite can be found in various parts of the world. Preventive measures, such as controlling mosquito breeding sites and using insecticide-treated bed nets, can help reduce the spread of the parasite and prevent the transmission of malaria.
Describe the trophozoite stage of Plasmodium vivax.
The trophozoite stage of Plasmodium vivax is one of the several stages of the parasite’s life cycle. During this stage, the parasite is actively growing and multiplying within the red blood cells of the human host.
The trophozoite stage begins after the merozoites, which are the offspring of the parasite, invade and multiply within the red blood cells. Once inside the red blood cell, the merozoites develop into trophozoites.
During the trophozoite stage, the parasite feeds on hemoglobin, which is a protein found in the red blood cells. The parasite breaks down the hemoglobin, releasing heme, which is toxic to the parasite. To avoid the toxic effects of heme, the parasite converts it into a crystalline substance called hemozoin, which accumulates within the parasite’s digestive vacuole.
As the trophozoite stage progresses, the parasite grows in size, and the red blood cell becomes enlarged and distorted. The trophozoite stage lasts for about 24 to 48 hours, depending on the species of Plasmodium.
After the trophozoite stage, the parasite enters the schizont stage, where it undergoes multiple divisions to produce new merozoites. The merozoites are then released from the red blood cells, and the cycle continues.
Understanding the different stages of the Plasmodium vivax life cycle is crucial in developing strategies to control and prevent the spread of malaria. Preventive measures, such as using insecticide-treated bed nets and taking antimalarial medication, can help reduce the transmission of the parasite and prevent the development of severe malaria.
Why is Plasmodium called Sporozoa?
Plasmodium is classified as a type of protozoa known as Sporozoa because of its unique reproductive mechanism. Sporozoa are a group of parasitic protozoans that reproduce through a process known as sporogony.
In the case of Plasmodium, the parasite undergoes a complex life cycle that involves both sexual and asexual reproduction. The asexual stage of the parasite’s life cycle occurs within the red blood cells of the human host, where the parasite multiplies through schizogony, a process where the parasite undergoes multiple divisions to produce new merozoites.
The sexual stage of the parasite’s life cycle occurs within the mosquito vector, where the parasite undergoes sporogony. During sporogony, the parasite divides to produce sporozoites, which are then transmitted to the human host when the mosquito bites.
The unique feature of sporozoans, including Plasmodium, is that they produce spores, which are specialized cells that enable the parasite to survive in harsh environments, such as outside the host’s body. The spores are resistant to heat, cold, and desiccation, allowing them to remain viable for extended periods.
The production of spores during sporogony is why the group is called Sporozoa. This feature distinguishes them from other protozoans that reproduce through binary fission or budding.
Which is known as the infective stage of Plasmodium vivax?
The infective stage of Plasmodium vivax is the sporozoite stage. Sporozoites are the form of the parasite that is transmitted from the mosquito vector to the human host during a mosquito bite.
When a mosquito carrying the sporozoites bites a human, the sporozoites are injected into the human’s bloodstream. The sporozoites then travel to the liver, where they invade and multiply within the liver cells. This is known as the exoerythrocytic stage of the parasite’s life cycle.
After a period of time, the sporozoites develop into merozoites, which are released into the bloodstream and invade the red blood cells. This is known as the erythrocytic stage of the parasite’s life cycle, during which the symptoms of malaria occur.
Understanding the infective stage of Plasmodium vivax is important in developing strategies to prevent the transmission of the parasite. Preventive measures, such as using insecticide-treated bed nets, wearing long-sleeved clothing, and using mosquito repellent, can help reduce the risk of being bitten by an infected mosquito. Additionally, antimalarial medication can be taken to prevent the development of the parasite if bitten.
What is the signet ring stage?
The signet ring stage is a stage in the development of Plasmodium vivax, a parasite that causes malaria. During the signet ring stage, the parasite is in its early trophozoite stage and is characterized by a small, ring-shaped structure within the red blood cell. The parasite appears as a small, pink or purple dot within the center of the red blood cell, giving it a signet ring appearance.
The signet ring stage is an important stage in the life cycle of the parasite because it is the beginning of the parasite’s asexual replication within the human host. During this stage, the parasite feeds on hemoglobin, a protein found in red blood cells, and begins to replicate.
As the parasite replicates, it undergoes multiple stages of development within the red blood cells, causing the cells to rupture and release the newly formed merozoites into the bloodstream. These merozoites can then invade other red blood cells, causing further infection and the development of the symptoms of malaria.
Identification of the signet ring stage is important in the diagnosis of malaria, as it is a characteristic feature of the early stages of infection. Blood smears are typically used to identify the presence of the parasite and the stage of infection. Early diagnosis and treatment of malaria are critical in preventing the development of severe complications associated with the disease.
- Menkin-Smith L, Winders WT. Plasmodium vivax Malaria. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538333/
- https://www.vivaxmalaria.org/p-vivax-malaria-an-introduction/lifecycle-of-plasmodium-vivax-malaria
- https://docs.google.com/file/d/0B0Izh6GcIA_DZ2RsYzRvbFlHRUU/edit?resourcekey=0-rSNhHnnTJzwTMz9HLZRqVA
- https://www.onlinebiologynotes.com/plasmodium-falciparum-morphology-life-cycle-pathogenesis-and-clinical-disease/
- https://www.biologydiscussion.com/protozoa-2/plasmodium-vivax-morphology-and-life-history-zoology/49295
- https://www.notesonzoology.com/protozoa/life-cycle-of-plasmodium-vivax-with-diagram-protozoa/5694
- https://www.cdc.gov/dpdx/resources/pdf/benchAids/malaria/Pvivax_benchaidV2.pdf
- https://www.cdc.gov/dpdx/malaria/index.html
- https://www.vivaxmalaria.org/p-vivax-malaria-an-introduction/lifecycle-of-plasmodium-vivax-malaria
- https://microbiologynotes.com/life-cycle-of-plasmodium-vivax/
- https://www.geeksforgeeks.org/plasmodium-life-cycle/
- https://en.wikipedia.org/wiki/Plasmodium_vivax
- https://microbeonline.com/asexual-life-cycle-of-plasmodium-falciparum/
- https://www.biologydiscussion.com/malaria/plasmodium/life-cycle-of-plasmodium-with-diagram-malaria-human-diseases-biology/82276
- https://www.vedantu.com/biology/plasmodium-vivax
- https://microbenotes.com/plasmodium-vivax-life-cycle/
good.