Pipetting Definition
Pipetting is the process of using a pipettor to measure and dispense small volumes of liquid. It is a fundamental technique used in many laboratory procedures, including preparing solutions, dispensing samples for analysis, and performing various types of assays.
When pipetting, the user first sets the desired volume on the pipettor by adjusting the thumb wheel or lever. Then, the pipettor’s tip is placed into the liquid, and the trigger or button is activated to draw up the liquid into the pipettor. The tip is then moved to the destination container and the trigger or button is activated again to dispense the liquid.
Accuracy and precision are important considerations when pipetting. Many modern pipettors are designed to be highly accurate and precise, with the ability to dispense volumes to within a few microliters of the set volume. However, it’s also important for the user to be properly trained in the use of the pipettor and to adhere to proper pipetting technique to ensure accurate and precise results.
Pipetting can also be done manually without using any instrument, it is called as manual pipetting but it’s not as precise as using a pipettor and also it’s more laborious.
What is the Purpose of Pipetting?
The purpose of pipetting is to measure and dispense small volumes of liquid with a high degree of accuracy and precision. This is critical for many laboratory procedures, including:
- Preparing solutions: Pipetting is used to accurately measure and dispense the correct volumes of reagents and other materials needed to prepare solutions. This is important for ensuring that the solutions are of the correct concentration and that they are consistent between different experiments or samples.
- Dispensing samples for analysis: Pipetting is used to accurately measure and dispense small volumes of samples for various types of analysis, such as in analytical chemistry, molecular biology or genetic engineering.
- Performing various types of assays: Pipetting is used in many types of laboratory assays, such as enzyme-linked immunosorbent assays (ELISAs) or polymerase chain reactions (PCRs). These assays require precise measurement and dispensing of reagents, enzymes, and nucleic acids, and accurate results relies on the precise and consistent pipetting of these reagents.
- Quality Control: Pipetting is also used in quality control procedures, where it is necessary to measure the volume of liquid products, such as pharmaceuticals and cosmetics, for compliance with regulatory standards.
- Research and Development: Pipetting is a critical technique for many different types of research, including in the fields of chemistry, biology, and medicine. It is used to measure and dispense the reagents, samples, and other materials needed for a wide range of experiments and studies.
What is Pipettors?
A pipettor, also known as a micropipette or pipette, is a laboratory instrument used to measure and dispense small volumes of liquid accurately. Pipettors come in a wide range of designs and sizes, and can be used to dispense volumes as small as a few microliters up to several milliliters. They are commonly used in chemistry, biology, and medical laboratory settings for tasks such as preparing solutions, dispensing samples for analysis, and performing various types of assays.
Pipettors can be classified into two main types: manual and electronic. Manual pipettors are operated by adjusting a thumb wheel or lever to set the volume to be dispensed, and then squeezing a trigger to release the liquid. Electronic pipettors, on the other hand, are operated by a button or joystick and use electronic controls to dispense liquid.
Pipettors can also be classified based on their volume range and the types of liquids they can dispense. For example, there are single channel and multi-channel pipettors, fixed volume or adjustable volume pipettors, and also pipettors that can handle viscous or volatile liquids.
There is also a class of pipettors called “Electronic pipettor” that are more precise, faster and ergonomic than the manual pipettors. They uses electronic motor and circuit to control the dispensing volume and also can be connected to a computer for data recording and analysis.
Types of Pipettors According to their operating principle
Manual and electronic pipettors are distinguishable based on their mode of operation. The piston in manual pipettors is manipulated with the thumb using an operation knob. Pipetting accuracy and precision are dependent on the operator’s skill. In electronic pipettes, the piston is propelled by a little electric motor. According to the parameters of the pipetted solution, various aspiration and expulsion speeds can be adjusted.
According to their operating principle, pipettors can be split into two major categories:
1. Air displacement“ pipettors
This variety of pipettes implements the so-called air cushion principle. There is a specific volume of air between the piston and the liquid being measured. The volume of solution aspirated or expressed with the piston may differ from the volume of air. It relies on the density and viscosity of the liquid, the surface wettability of the tip with the pipetted liquid, temperature, atmospheric pressure, and other factors. Therefore, every pipettor must be calibrated frequently.
Air displacement pipettors can be built as a single channel (for pipetting of a single volume at a time) or as a multi channel (most frequently with eight or twelve channels). Multichannel pipettors are designed to pipette the same volume of liquid simultaneously into multiple wells of a microtitration plate. Each channel has its own piston, therefore it is not required to use all eight or twelve channels (i.e. less tips can be attached to the pipette).
It is possible to set the volume of a micropipette, or it is designed for a fixed volume. Using an adjustable screw or knob, volumes can be set either separately (through swapping of plug-in modules) or continuously in a specified range (e.g., 10–100 L) At the bottom of a micropipette is a detachable tip. At the opposite end of the pipettor is the control knob for the piston placed into the cylinder.
2. Positive displacement“ pipettors
Without an air cushion, liquid is aspirated into the tip; the piston is in contact with the measured liquid. The liquid is then given in a single or many stages (in case of so called “steppers”). This type of pipettors is appropriate for highly viscous or aqueous liquids and repetitive pipetting.
Types of Pipettors According to their functions
There are many different types of pipettors available, each with their own unique features and capabilities. Some of the most common types of pipettors include:
- Manual pipettors: These are the most basic type of pipettors, and are operated by adjusting a thumb wheel or lever to set the volume to be dispensed, and then squeezing a trigger to release the liquid. They are typically less expensive than other types of pipettors, but also less precise and may be less comfortable to use for extended periods.
- Electronic pipettors: These pipettors use electronic controls to dispense liquid, and are operated by a button or joystick. They are typically more precise and accurate than manual pipettors, and can be faster and more comfortable to use, since they can be programmed with different volume settings. They can also be connected to a computer for data recording and analysis.
- Positive displacement pipettors: These are a special type of pipettors that are designed to ensure that a precise and accurate volume of liquid is dispensed, regardless of variations in liquid viscosity, density, or surface tension. They use a small piston or other mechanism to push the liquid out of the pipette tip.
- Multichannel pipettors: These pipettors have multiple channels (usually 8, 12 or more) that allows multiple volumes of liquid to be dispensed at the same time, increasing the efficiency of the process. They are commonly used in high-throughput applications such as PCR and ELISA, where many samples need to be processed at the same time.
- Single Channel, Adjustable volume pipettors: These pipettors can adjust the volume of liquid to be dispensed. They are commonly used when working with multiple samples or different solutions that require different volumes to be dispensed.
- Variable Volume pipettors: These pipettors allow the user to adjust the volume of liquid that is dispensed within a range of volumes. They are commonly used when working with multiple samples or different solutions that require different volumes to be dispensed.
- Fixed Volume pipettors: These pipettors have a fixed volume that cannot be adjusted, making them less versatile but also more reliable for applications where a specific volume is always required.
Proper Pipetting Technique / Operating a Pipettor
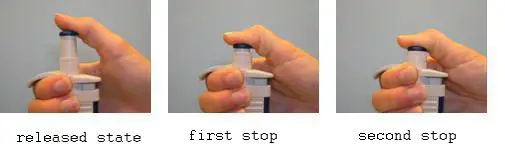
1. Forward Pipetting technique
This is the most commonly employed method. In forward pipetting, a precise volume of liquid is aspirated to the tip of the pipette and then transferred to a new receptacle. This method is advised for pipetting diluted aqueous solutions, buffers, and diluted acids and bases.
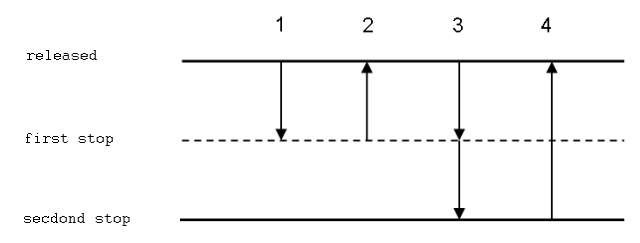
Procedure of Forward Pipetting technique
- Connect a tip to the pipette. Press the button corresponding to the first stop (it is necessary to overcome a slight resistance).
- Submerge the tip approximately 1-2 mm below the solution level. Slowly release the button to aspirate the solution to the tip. Slow aspiration prevents tubulences that would result in the creation of aerosol and dissolved gas bubbles. The ideal velocity depends on liquid characteristics (density, tension of vapours and viscosity).
- Take care not to inhale air bubbles (e.g. when the piston is released to quickly or if the tip is not attached properly).
- If the thumb is totally removed from the button once it has reached the released position, greater precision can be achieved.
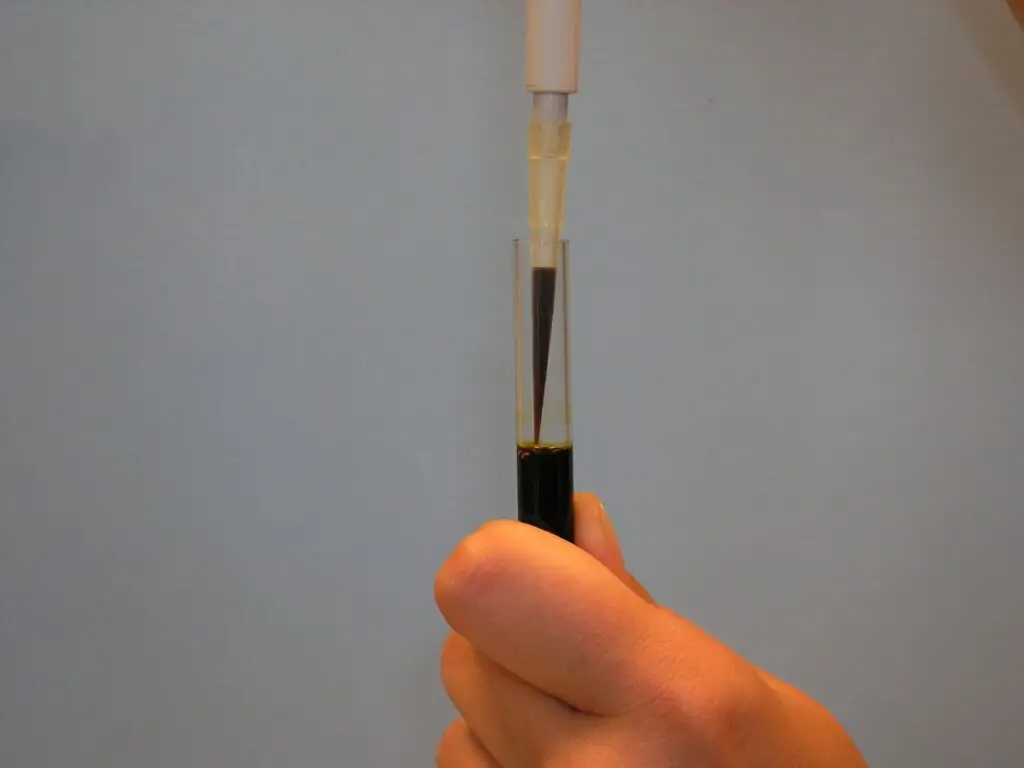
- Slowly remove the liquid’s cap. Rapid removal may result in the loss of some content. Wait several seconds before removing the tip, especially when working with big volumes (500–5000 μL).
- When necessary, wipe the external surface of the tip with a tissue cloth to remove any drips. Never touch the tip’s aperture, as the cloth would absorb some of the transferred volume.

- During liquid distribution, the tip should contact the vessel wall at an angle of 10–45 degrees. It should be just above the level of any existing liquid in the container. Press the button for the initial stop. Wait approximately 1 second, then swiftly push the button to the second stop (a stronger resistance can be felt). No liquid should remain in the tip and none should splash onto the walls.

- Maintaining the button in the second position, withdraw the tip while maintaining contact with the vessel wall. Now you may let go of the button.
As a thin film of the pipetted solution stays on the inner walls of the tip during forward pipetting, a tiny volume inaccuracy is introduced. In other words, the outlined technique results in the delivery of a slightly smaller quantity than required. This error relies on the qualities of the liquid being measured and the tip. If the inner wall of the tip is pre-rinsed with the measured liquid, the mistake can be avoided. In practise, liquid is initially aspirated to the tip. Then, rather than being supplied to a new vessel, it is returned to the stock vessel. Now, a very thin layer of pipetted liquid covers the tip’s inner wall (it is usually invisible). Pipetting according to the preceding directions follows (without changing the tip). As the remaining volume of liquid in the tip is nearly constant, the precise volume specified is measured.
2. Reverse Pipetting technique
In the reverse approach, more solution is aspirated to the tip. Then, a volume of exaxt is supplied (and some liquid remains in the tip). This method is appropriate for measuring extremely viscous or volatile liquids, biological fluids, foamy solutions, and extremely small quantities.
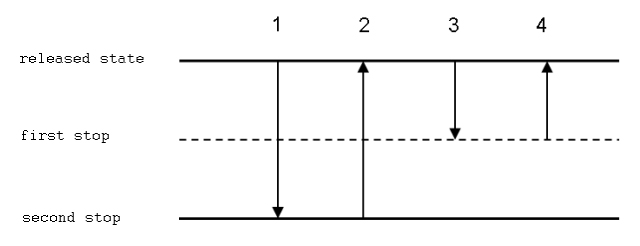
Procedure of Reverse Pipetting technique
- To advance to the second stop, press the button to the second position.
- Dip the tip 2–5 mm below the solution’s surface. Ascend the solution to the tip gradually.
- Slowly remove the tip from the solution. If required, clean the external surface of the tip to remove any drips.
- Deliver the liquid to a new container by pushing the button just to the first stop. The tip should similarly contact the vessel’s wall as in the forward technique.
- Remove the tip from the vessel while the button is in the first position.
- The residual liquid in the tip may be returned to the stock vessel or discarded.
3. Pipetting of heterogenous samples
This method is appropriate for heterogeneous samples such as whole blood. In this scenario, pre-rinsing the tip before pipetting is difficult.
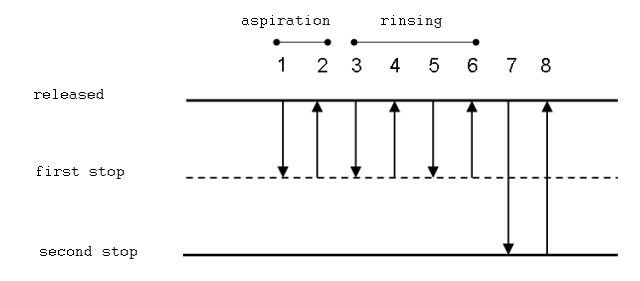
Procedure
- Push the button to the first stop and dip the tip approximately 2–5 mm below the level of the solution.
- Release the button gradually. The sample is aimed for the apex.
- Remove the tip slowly from the solution and wipe drips from the tip’s outer wall.
- The tip is dipped in the target solution.
- Press the button to its initial stop, and then slowly let it go. The solution will be sought after. Repeat this process until the inner wall of the tip is clean, without removing it from the solution.
- Place the tip above the level of the solution while touching the vessel’s wall and press the second stop button.
- Hold the second stop button and withdraw the tip from the vessel.
4. Repetitive pipetting
This method is utilised for measuring the same volume of a liquid into multiple test tubes or multiple wells on a microtitration plate. Similar to reverse pipetting, steps two through four are repeated multiple times.
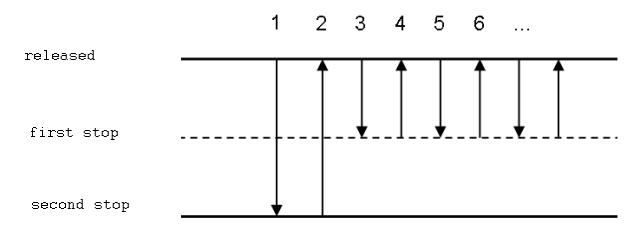
This method is intended for pipetting the same volume multiple times. Only compatible with electronic or repeater pipettes.
- Press the button to the second position.
- Immerse the tip to a depth of 1 cm in the solution, and then slowly release the operating button. Eliminate the tip from the liquid and wipe it on the reservoir’s rim to remove extra liquid.
- By gently depressing the button to the first stop, the liquid is dispensed into the receiving container. Maintain this button position. Some remaining liquid should not be dispensed from the tip.
- Repeat actions 2 and 3 to continue pipetting.
How to Pipetting Whole Blood?
Steps 1 and 2 of the forward approach are used to fill the tip with blood (do not pre-rinse the tip). Wipe the tip with care with a clean, dry cloth.
- Dip the probe’s tip into the blood and press the button to the initial stop. Ensure that the tip is sufficiently submerged.
- Slowly return the operational button to the ready position. This action will cause blood to fill the tip. Do not remove the solution’s tip.
- Press the button to the first stop and slowly release it. Repeat until the internal wall of the tip is free of debris.
- Press the button to the second stop and empty the tip entirely. To remove the tip, slide it along the vessel’s wall.
- Release the button to its ready position.
Positive displacement pipetting
Positive displacement pipetting is a technique that uses a special type of pipettor called a positive displacement pipette. These pipettors are designed to ensure that a precise and accurate volume of liquid is dispensed, regardless of variations in liquid viscosity, density, or surface tension.
Positive displacement pipettes work by first filling the pipette tip with a liquid. Then, when the liquid is dispensed, a small piston or other mechanism is used to push the liquid out of the pipette tip. This ensures that the liquid is displaced in a precise and consistent manner, regardless of variations in the liquid’s properties.
Positive displacement pipettes are often used for dispensing viscous or volatile liquids, or for applications where high accuracy and precision are required. They are also commonly used in clinical and pharmaceutical laboratories, as well as in the food and beverage industry, as these industries require highly accurate and precise measurement.
There are different types of positive displacement pipettes, they can be mechanical or electronic, single or multi-channel, fixed volume or adjustable volume. Electronic positive displacement pipettes are more accurate and precise and can be connected to a computer for data recording and analysis.
In summary, positive displacement pipetting is a technique that uses a special type of pipette, called positive displacement pipette, which is designed to ensure precise and accurate volume of liquid dispensing regardless of variations in liquid viscosity, density, or surface tension. These pipettes can handle viscous or volatile liquids, and are commonly used in industries that require high accuracy and precision in liquid measurements.
What is ergonomic pipetting?
Ergonomic pipetting refers to the design of pipettors and other laboratory equipment that takes into account the comfort and safety of the user. The goal of ergonomic design is to reduce the physical strain and risk of injury associated with repetitive tasks, such as pipetting.
Ergonomic pipettors are designed to be easy to hold and use for extended periods of time. They may have a more comfortable grip and a lightweight design to reduce hand fatigue. They can also have a reduced trigger force, so that less effort is required to dispense liquid. Some pipettors have also designed with an electronic pipetting which reduces the hand movement.
Ergonomic pipettors may also have an adjustable finger rest and trigger position, so that the pipettor can be customized to the individual user’s hand size and shape. Additionally, they may have other features such as an electronic volume display, automatic tip ejector and digital tip recognition to make the pipetting process more efficient and user-friendly.
Ergonomic design can also apply to other laboratory equipment such as laboratory workstations, chairs, and laboratory furniture. This is to ensure that the work area is set up in a way that promotes good posture, comfort, and safety.
In summary, ergonomic pipetting refers to the design of pipettors and other laboratory equipment that takes into account the comfort and safety of the user by reducing the physical strain and risk of injury associated with repetitive tasks such as pipetting. This is done by designing pipettors that are easy to hold and use for extended periods, lightweight and have a comfortable grip, reduced trigger force, adjustable finger rest and trigger position, and other features that make the pipetting process more efficient and user-friendly. Ergonomic design can also apply to other laboratory equipment such as laboratory workstations, chairs, and laboratory furniture to ensure that the work area is set up in a way that promotes good posture, comfort, and safety.
Pipetting Guidelines For Selected Compounds
A. Body Fluids
Whole Blood
- Pipette + tip combination: Choose an air displacement pipette and a standard or wide aperture tip for your pipette and tip combination.
- Method: utilise the entire blood pipetting method. If high accuracy is required, reverse pipetting should be employed.
- Blood can potentially remain in the tip and on the surface. Before dispensing, wipe the tip against the vessel’s rim to remove extra liquid from the tip.
Serum
- Pipette + tip combination: Choose an air displacement pipette and a standard or wide aperture tip for your pipette and tip combination.
- Method: utilise the entire blood pipetting method. If high accuracy is required, reverse pipetting should be employed.
- On occasion, residual serum might be seen on the outside surface of the tip. Before dispensing, wipe the tip against the vessel’s rim to remove extra liquid from the tip.
B. Only Fluid
Glycerol
- Pipette + tip combination: Choose an air displacement pipette and a standard or wide aperture tip for your pipette and tip combination.
- Technique: For precise results, employ the reverse pipetting method.
- Due to the development of air bubbles, oily liquids are challenging to pipette. Very slow filling is required to prevent air bubbles. Before dispensing, wipe the tip against the vessel’s rim to remove extra liquid from the tip. The use of a pipette and tip with positive displacement is also advantageous for pipetting glycerol.
Tween 20, 10% solution
- Pipette + tip combination: Choose an air displacement pipette and a standard or wide aperture tip for your pipette and tip combination.
- Technique: utilise the reverse pipetting technique.
- Tween has a very high viscosity; it should be diluted to a 10% solution for easy pipetting. In any event, pipetting will not be precise; liquid will remain in the tip. Aspiration and administration must be performed slowly. The use of a pipette and tip with positive displacement is also recommended for pipetting Tween 20.
Bronidox L, 10% (preservative)
- Pipette + tip combination: Choose an air displacement pipette and a standard or wide aperture tip for your pipette and tip combination.
- Technique: utilise the reverse pipetting technique.
- Bronidox L is extremely viscous; aspiration and dispensing should be performed gently or with a positive displacement pipette and tip.
C. Salt Solution
10 x PBS, 0.1M NaCl, 3M
- Choose an air displacement pipette and a standard tip for the pipette-and-tip combination.
- Utilize the forward pipetting technique for the procedure. Pre-wetting the tip prior to aspiration improves precision.
D. Concentrated Acids And Bases
H2SO4
- Pipette + tip combination: Choose a pipette with an air displacement mechanism and a filter tip.
- Technique: Utilize the forward pipetting technique for the procedure.
NaOH
- Pipette + tip combination: Choose a pipette with an air displacement mechanism and a filter tip.
- Technique: Utilize the forward pipetting technique for the procedure.
- Some acids and bases readily evaporate (e.g. trifluoroacetic acid). Do the pipetting relatively swiftly to limit vapour production.
E. Nucleic Acids
Wide orifices should be used to prevent mechanical shearing of genomic DNA.
- Pipette + tip combination: Choose either an air displacement pipette with a filter tip or a positive displacement pipette with a tip.
- Technique: Utilize the forward pipetting technique for the procedure.
- Wide orifice tips can be utilised to prevent mechanical shearing of genomic DNA.
F. Volatile Compounds
- Pipette + tip combination: Choose either an air displacement pipette with a filter tip or a positive displacement pipette with a tip.
- Technique: Utilize the forward pipetting technique for the procedure.
- Notice: 1.) To acquire reliable results, calibrate the pipette with the volatile substance you intend to pipette. If you are using pipettes with air displacement, aspirate and dispense the liquid many times while maintaining the tip in the liquid. By doing so, the air inside the pipette will be saturated with vapour of the volatile substance. When utilising air displacement pipettes, pipette quickly to avoid evaporation. Since the piston tip is in direct contact with the liquid, it is suggested to utilise positive displacement pipettes for extremely volatile substances.
How to Prevent Cross-Contamination?
Pipette-to-sample
A contaminated pipette or contaminated tips might result in sample contamination.
Prevention:
- Use sterile tips or filter tips, and autoclave the pipette if feasible.
- Replace the pipette tip after pipetting each sample.
Sample-to-pipette
Samples or aerosols derived from samples can enter the pipette’s cone.
Prevention:
- When pipetting, keep the pipette vertical to prevent liquid from entering the pipette body.
- Slowly release the press button.
- To avoid aerosol contamination, use filter tips or use a positive displacement pipette and tips.
- Pipettes are stored vertically.
Sample-to-sample (carry-over)
The remnants of sample A can potentially contaminate sample B within the tip, leading to an inaccurate test result. Prevention:
- Replace the tip between each sample.
- If you feel that your pipette is contaminated, you should sterilise or clean it.
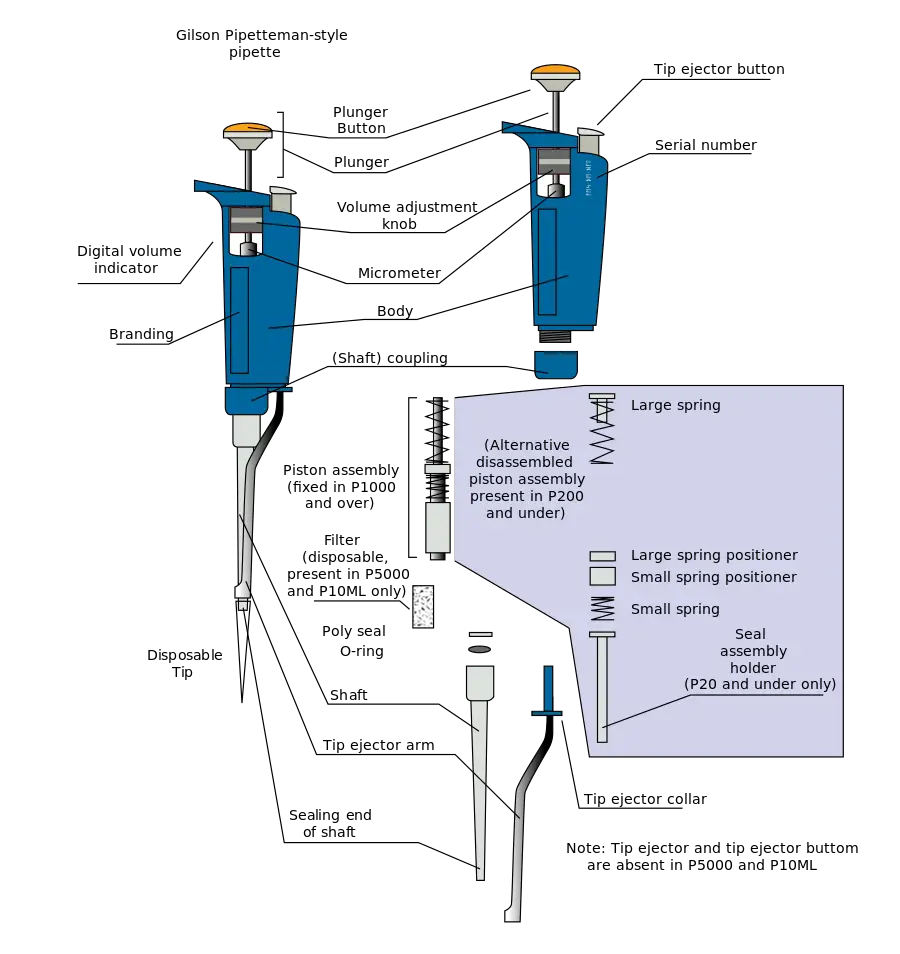
How to Select Tips for Your Pipette?
The Tip is one of the most crucial aspects of proper Pipette measuring. Pipette and Tip constitute a System. Those manufactured jointly to assure total compatibility between Pipettors and Pipettor Tips offer the finest pipetting outcomes.
Numerous tips on the market appear to be of high quality at first glance, but a closer examination reveals that their prices are proportional to their quality. Many of the inexpensive tips may contain flashes, protrusions, scratches, air bubbles, bending, or contaminants. All of these factors affect Pipetting outcomes. For instance, a non-straight pipette tip may result in a 10% inaccuracy in pipetting accuracy (ISO 8655-2). Furthermore, if the tip orifice is not precisely centred, liquid dispensing may be considerably affected. If premium grade pure polypropylene is not utilised or if the mould and processing are not up to the highest requirements, a portion of the liquid remains within the tip as a droplet, resulting in a flawed Pipetting outcome.
Guidelines For Selecting Tips
- Designed to fit the Pipettor in use: It is recommended to utilise both the manufacturer-recommended tips and high-quality Unifit tips.
- Produced from premium virgin polypropylene
- without dust or particles
- Consistent in size and structure
- Precisely centred for the tip orifice.
- Free of metals such as cadmium
- Completely sealed: A good tip cone match is necessary for a perfect seal and tip expulsion.
- Devoid of flaws, flashes, or protrusions.
- Highly chemical resistant
- Highly thermally stable
- Autoclavable or already sterilised for sterile purposes
- Traceable: Each tip contains lot numbers and mould cavity identifying markers.
How to Calibrate Your Pipettes?
Calibration of Pipettes
Pipette calibration entails calculating the difference between the dispensed volume and the desired volume. Adjustment entails modifying the pipette such that the dispensed volume conforms to specified parameters. During factory calibration, performance is evaluated using various weighings at the maximum volume of the range as well as the minimum volume or 10% of the maximum volume, whichever is greater. When purchasing a Pipette, you should select one that can be recalibrated and adjusted for varying temperatures and viscosities of liquids.
Calibration of Pipettes in a Quality System
The primary purpose of pipette calibration in a quality system is to guarantee that measurements are performed with the desired precision. Frequently, error limits are derived from the manufacturer’s requirements, despite the fact that significantly less precision is necessary to complete the task. If these limitations are difficult to achieve or vary, another alternative is to establish the limits in accordance with recognised standards (DIN 12650 or ISO 8655). However, if the laboratory job demands the maximum degree of precision, the manufacturer’s specifications should be followed. Every user should specify their own restrictions based on the application and the surrounding environment.
Device Requirements And Test Conditions
Balance: The scale graduation value of the balance should be selected based on the pipette volume used.
| Volume range | Readable graduation |
| Under 50 vl | 0.001 mg |
| Above 50 vl | 0.01 mg |
Note: check the calibration of your balance regularly using known weights.
Test liquid:
Grade 3 water, distilled or deionized, adhering to ISO 3696. Before calibration, the test water is maintained in the calibration room for at least two hours to achieve equilibrium with the test room conditions.
Test room:
The tests are conducted in a draft-free environment with a constant temperature of 20C to 25C (+/- 0.5C). It is recommended that relative humidity be above 45%. To minimise the effect of evaporation loss, the air humidity should be as high as possible, especially for volumes under 50 vl.
- The pipette, the water, and the room’s ambient temperature should all be the same.
- One to three repetitions of pre-wetting a new tip will increase its precision.
- Always pipette water from a reservoir; never return it to the scale.
- Check the calibration at least once per year, depending on the frequency of usage and the application. A three-month interval is recommended for daily use.
Procedures To Check Calibration
General Pipette Calibration Procedure
Incoming Inspection procedure
- Each Pipette is carefully unpacked and inspected for visible damage upon shipment. Any observable damage is immediately reported back to the consumer. The customer will be informed if the damage is repairable and of any additional costs. Customer will issue a new Work Order to cover Repair costs.
- Each Pipette is then scanned for radioactive contamination. Any radioactive contamination identified is promptly disclosed to the customer, and the pipette is rejected.
- All customer Pipettes are tallied and compared to the reported quantity on the given Work Order. Any quantity differences are immediately communicated to the consumer. Customer will have the option to discover any missing quantities; otherwise, the Work Order must be changed and Customer must sign the amendment.
- Each Work Order will be kept in an own internal storage box until Calibration is planned.
- For every Work Order, a unique Job ID number is assigned.
- The customer information is compared to the information on file and, if necessary, rectified. If this is a New Customer, a file containing customer information will be created.
- All Pipettes are entered into the Cal Lab database under a designated Job ID number, and each instrument is assigned an ID number.
Pre-calibration procedure
- As Found data are gathered and recorded prior to Calibration, as well as any Pipette modifications.
- Each Pipette will be examined at the minimum and maximum range points given by the manufacturer. Ten tests were conducted at each location.
- As part of Preventive Maintenance, each Pipette is dismantled in accordance with the manufacturer’s schematics and carefully cleaned using the corresponding chemical solutions.
- In most situations, the piston is polished, and the piston seal and o-ring are replaced, along with other damaged and dysfunctional parts.
- Each Pipette is reassembled according to the manufacturer’s drawings and procedures upon completion. Pipette is visually examined for functionality once again.
Calibration procedure
- Before any modifications are made, each Pipette is triple-checked at the beginning of the Calibration procedure. If the Pipette fails, the Calibration is adjusted as necessary. Following corrections, each instrument will be calibrated at the lowest and highest range points, with ten tests at each test point.
- Calibration Information will be recorded and reported on a Calibration Report.
- Calibration reports are generated for each Pipette in addition to a Calibration Summary for the entire project. Technicians who are qualified sign reports.
- Create and attach a Calibration Label to each Pipette.
- All Pipettes were compared to both customer-supplied and laboratory-generated documentation.
- The QC Supervisor examines the performance of randomly picked Pipettes.
- If an error is identified, the entire Job is rechecked.
- The Pipette is carefully wrapped and delivered to the customer with all Calibration Documentation enclosed when the QC Supervisor approves the job.
Calibration
- Before calibration, the pipette is kept in the calibration room for at least two hours to achieve equilibrium with the test room conditions.
- The pipette is examined at the maximum volume (nominal volume) as well as the lowest volume or 10% of the maximum volume, whichever is greater.
- With both volumes, ten pipettings are conducted in succession.
- Calculate the accuracy and precision using the formulas provided in the next section. Pipette calibration is accurate if the computed values are within the limitations specified in the Instructions for usage booklet. If not, the pipette must be readjusted to a smaller volume and rechecked.
Sources of pipette error
- Volatile liquids evaporate. Utilize in dense air.
- High density liquids sink. Change method.
- Viscous fluids adhere to the tip. Change method.
- Hydrophilic tips are liquid-retaining. Wetted tip.
- Immersed tips travel through liquid. Immerse 2-3mm.
- Angled pipettes take up more liquid. Keep vertical.
- Surface tension diminishes precision. Change method.
- Warm and cool liquids Pipette liquids at room temp.
- The human element. Practice and watch technique.
Procedures To Adjust The Pipette
Manual pipettes
- Adjustments are made at a reduced volume.
- Insert the pipette’s included service tool into the holes of the calibration nut at the top of the handle.
- Turn the service tool either clockwise or anticlockwise to increase or reduce the volume.
- Check the calibration as mentioned above after adjusting.
Electronic pipettes
- Due to major variances in calibration procedures, please adhere to manufacturer-specific recommendations.
Formulas For Calculating Results
Conversion of mass to volume
V = (w + e) x Z
V = volume (vl)
w = weight (mg)
e = evaporation loss (mg)
Z = conversion factor for mg/vl conversion
With modest volumes, evaporation loss might be severe. To calculate mass loss, pour water into the weighing vessel, record the reading, and start a stopwatch. Determine the drop in the reading over the course of five seconds. Compare this to the time for pipetting. Typically, the pipetting period is five seconds and the mass loss can reach two milligrammes. If a vessel is equipped with an evaporation trap or cover, evaporation correction is unneeded.
Calculate the density of water suspended in air at the test temperature and pressure using the conversion factor Z.
Accuracy (systematic error)
The accuracy of a pipette is the difference between the dispensed volume and the specified volume.
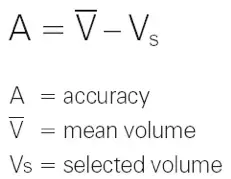
Accuracy is expressed on the calibration certificate as a relative value:
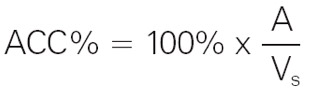
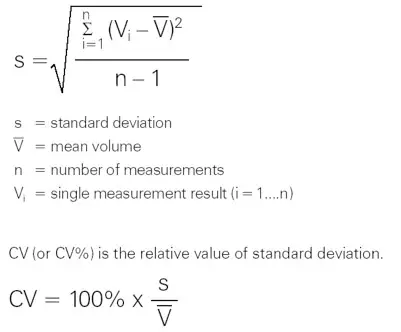
Precision (random error)
Precision refers to the pipettings’ repeatability. It is denoted by the standard deviation (s) or coefficient of variation (cv). In addition to the pipette’s characteristics, laboratory procedures and user experience are the primary elements that influence precision.
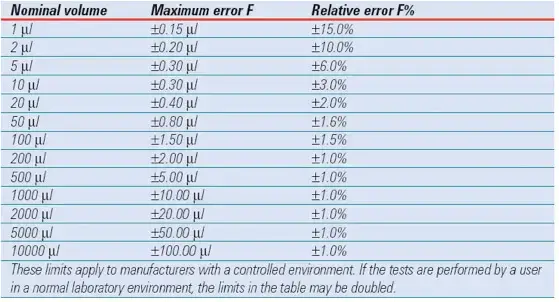
Error according to DIN 12650 (F-value)
The DIN standard does not give individual limits for accuracy and precision, but uses a combined error limit: the F-value.
F = | A | + 2 x s
The relative F-value is calculated:
F% = | A% | + 2 x cv
The error limit according to DIN 12650 for single-channel air displacement pipettes is displayed in Table 2. These parameters are doubled for multichannel pipettes. The nominal volume of variable volume pipettes is the maximum volume. The absolute vl limit of the nominal volume is applicable to all specified volumes within the volume range.
For example, the error limit for a 20-200 vl pipette is 2.0 vl for each specified volume. If the pipette’s nominal volume falls between those listed in the table, the relative error limit F% of the closest volume is utilised. If the nominal volume falls precisely between the two volumes in Table 2, the relative error limit F% for the lower volume is utilised.
Best Practices
Ensuring optimum performance
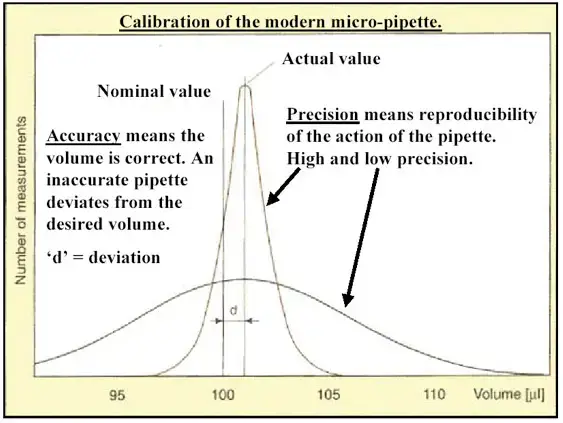
Pipetting without errors demands both precision and accuracy. These specifications, which are the primary quantitative characteristics for measuring pipette performance, can be affected by a variety of variables.
What are precision and accuracy?
For instance, when the volume is set to 20 vl:
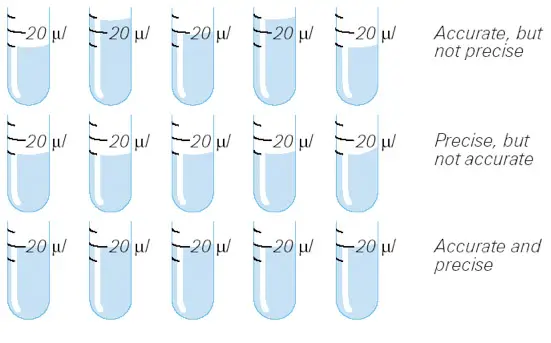
- Accurate, but not precise: The mean volume is the correct (predetermined) value, although individual pipettings deviate from the predetermined volume.
- Precise, but not accurate: There is no variance between the individual pipettings, however the average volume differs from the desired volume.
- Accurate and precise: The mean volume is the specified volume, and there is no variance between individual pipettings.
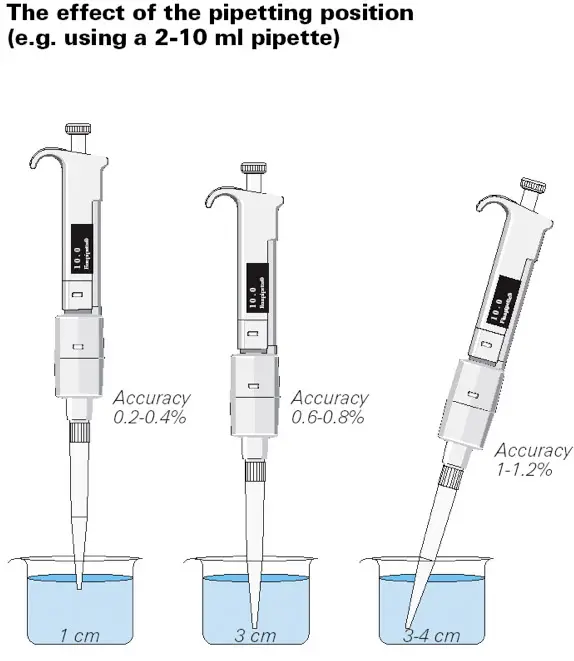
- Pipette should be held vertically with the tip submerged approximately 1 centimetre into the liquid.
- Pipette should be held vertically with the tip submerged approximately 3 cm into the liquid.
- Position the pipette at a 30 to 40 degree angle, with the tip buried 3 to 4 cm into the liquid.
Autoclaving (Sterilization)
Please adhere to these directions carefully to prevent damage to pipette tips and pipettes.
- Autoclave tips at 121 degrees Celsius for twenty minutes. The tips are moist immediately upon autoclaving. Before using the tips, allow the moisture to evaporate, ideally overnight.
- Some digital model Pipettes can be autoclaved in one piece for 20 minutes at 121°C (248°F). The single-channel variants’ tip cone modules can also be autoclaved.
- Before use, the pipette must be chilled at room temperature for at least two hours following autoclaving.
- Check the pipette’s calibration after each autoclave treatment.
- Autoclaving has a limited spectrum of action and will not, for instance, degrade RNA. It is also crucial to remember that certain pipette components, such as the piston and the handle, cannot be autoclaved without compromising precision and accuracy.
Ten recommended steps for improving pipetting technique
- Temperature fluctuations (≅2%) may impair the precision of your pipetting if the lab environment is not as well controlled as possible. Before aspirating liquids, they should be brought to room temperature wherever possible.
- Equilibrate the air within the tip with the liquid by wetting the tip beforehand (aspirate 3 times and dispense to waste). This improves the precision of your aspiration and the uniformity of repeat reagent or sample transfers.
- Hold the pipette vertically, and never at an angle more than 15 degrees. This will prevent liquids from entering the pipette shaft and causing corrosion. A pipette with a damaged shaft may be inaccurate and unusable.
- Maintain a tip immersion depth between 2 and 5 mm and a vertical position during the sample aspiration process. If the tip is immersed too deeply or too shallowly in the liquid, the accuracy may decrease by up to 1%.
- Apply constant, even force to the pipette plunger and prevent abrupt or jerky motions. Inability to modulate plunger speed may result in a 0.5% decrease in pipetting precision.
- After aspirating, pause for one to three seconds while keeping the tip submerged in the liquid. This will provide ample time for the liquid to go into the pipette tip. This practise will also reduce the frequency of systematic errors.
- Remove the pipette tip from the container without touching the container’s walls. This will ensure that no volume of sample or reagent is lost prior to dispensing into the final container.
- Remember to wipe the dispensing tip against the container’s inner wall after use. This expels any liquid that may have remained in the tip. Failing to do so can result in an approximate 3% reduction in accuracy.
- Reduce pipette handling to prevent the transfer of body heat to the pipette. As noted in steps one and two, a continuous environment increases precision.
- Inspect pipette tips routinely before loading or aspirating liquids. Never utilise damaged, bent, or infected tips, since they pose a substantial risk of error. Inaccurate tips can reduce accuracy by up to 10%.
- Always adjust the volume of a mechanically adjustable pipette in a clockwise manner. If you are decreasing the volume, you may travel a half-turn past the target value before moving back in the clockwise direction.
- Always use ergonomic pipettes to minimise user pain. Working with a pipette that is comfortable and maintaining right posture will help you acquire proper pipetting technique and reduce the chance of harm.
- Always use low-retention pipette tips of top quality that fit perfectly on the nose cone of your pipette. Improperly loaded pipette tips have a poor fit that can reduce the accuracy of pipetted quantities by up to fifty percent.
Prevent Pipetting Errors
- Daily cleaning and inspection of pipettes: The accuracy of a pipette is dependent on its lack of contamination, so it is essential to clean it before and after each usage. Before beginning work, make sure to visually inspect your pipette to confirm that it seems to be in working order and is not damaged.
- Six monthly maintenance on your pipette: Depending on how precise your results must be, you should get your pipette serviced approximately every six months. This requires thorough pipette disassembly for recalibration and replacement of broken parts. If your lab does not service its own pipettes, you must send them to a reliable “pipette doctor” such as Pipettes.com to assure reliable service.
- Know correct pipetting procedures: If you’re new to pipetting or need a refresher, make sure you’re using a pipette according to the following guidelines:
- Employ a leisurely and fluid motion.
- Wet the pipette tip beforehand.
- When drawing in liquid, hold the pipette upright, and when dispensing, hold it at a 45-degree angle.
- Slightly submerge the tip in the liquid during inhalation.
- Touch off the pipette on the container’s sidewall.
- Use the proper pipette: It is essential to utilise a pipette with a volume setting that corresponds to the volume you intend to aspirate and dispense. If there is a slight variation between the minimum volume on the pipette and the volume being tested, your test will be more accurate. Example: A 1mL pipette would be inappropriate for dispensing 15 microliters, but a 20 microliter pipette would be optimal.
- Account for environmental conditions: Consider the constancy of temperature, pressure, and humidity, among other variables. As liquids are subject to all forms of environmental changes, the accuracy of your results will be affected by an environment that is inconsistent. Before recording findings, it is crucial to account for sample temperature and dispense all liquids only once.
- Let your pipette adjust to the environment: It is advised that you let your pipette and other testing equipment to acclimatise to new environmental conditions and temperatures. Consequently, less contextual variables will influence your outcomes.
- Keep your pipette safe: Your pipette is the key to accurate results; do not endanger it! Never hold the pipette when it is not in use. Never place it on the laboratory bench. Keep it always on a stand. Always keep it upright when storing. By adhering to these pipetting procedures, you ensure that your pipette is free of contaminants and in excellent condition.
- Take a break: Take a break if you’re in the middle of what seems to be an endless test (if possible). Your outcomes will reflect the state of your mind, muscles, and eyes; weariness can lead to sloppiness and errors. Remember to maintain proper posture and keep your elbows in and your arms out in front if you are unable to take a break while taking a time-sensitive test.
- Use a different tip each time: The seemingly unlimited number of pipette tips in your lab is so that you can use a different tip for each liquid you test. A tip that is repeatedly used for various chemicals and tests is subject to cross-contamination. A pipette servicer must change the pipette tip prior to testing a new chemical, similar to when a doctor changes his or her gloves before interacting with a new patient.
- Practice, practice, practice: As with any endeavour, the more experience a person has, the more likely the outcome will be favourable. A pipette technician with years of experience in calibration will assure the precision, dependability, and knowledge required for effective performance evaluation and maintenance.
Applications of Pipetting
Pipetting is a widely used technique in many laboratory procedures, and has a wide range of applications in various fields, including:
- Chemical Analysis: Pipetting is used in many different types of chemical analysis, including in analytical chemistry, where it is used to measure and dispense small volumes of samples and reagents.
- Biology and Medicine: Pipetting is used in many applications in the field of biology and medicine, such as in genetic engineering, molecular biology, and biochemistry, where it is used to measure and dispense small volumes of enzymes, nucleic acids, and other biological reagents.
- Pharmaceuticals: Pipetting is used to measure and dispense the precise volumes of liquid used in the development and production of pharmaceutical products. It is also used to measure the volume of liquid products, such as syrups, for compliance with regulatory standards.
- Food and Beverage Industry: Pipetting is used to measure and dispense the precise volumes of ingredients and additives used in food and beverage products, as well as to measure the volume of liquid products for compliance with regulatory standards.
- Environmental Analysis: Pipetting is used in many applications in the field of environmental analysis, including for the measurement of pollutants and contaminants in air, water, and soil samples.
- Biotechnology: Pipetting is used in many applications in the field of biotechnology, such as cell culture and protein purification, where it is used to measure and dispense small volumes of reagents and biological samples.
- Quality Control: Pipetting is also used in quality control procedures, such as checking the volume of liquid products, to ensure compliance with regulatory standards.
- Research and Development: Pipetting is a critical technique for many different types of research, including in the fields of chemistry, biology, and medicine, where it is used to measure and dispense the reagents, samples, and other materials needed for a wide range of experiments and studies.
Advantages of Pipetting
Pipetting is a widely used technique in many laboratory procedures, and has several advantages, including:
- Precision and accuracy: Pipettors are designed to be highly accurate and precise, with the ability to dispense volumes to within a few microliters of the set volume. This makes them ideal for applications where precise measurements are critical, such as in analytical chemistry or molecular biology.
- Consistency: Pipettors can be used to dispense consistent volumes of liquid, even for large numbers of samples. This makes them useful for high-throughput applications, such as in genetic engineering or biochemistry, where many samples need to be processed at the same time.
- Versatility: Pipettors are available in a wide range of designs and sizes, and can be used to dispense volumes as small as a few microliters up to several milliliters. This makes them suitable for a wide range of applications, such as in chemical analysis, environmental analysis, or quality control.
- Safety: Pipettors are designed to minimize the risk of spills or splashes, and most have a number of safety features, such as an automatic tip ejector, to ensure the safe handling of liquids.
- Ergonomics: Many pipettors are designed to be ergonomic, which means they take into account the comfort and safety of the user. The goal of ergonomic design is to reduce the physical strain and risk of injury associated with repetitive tasks such as pipetting.
- Efficiency: Pipetting can be done with the help of electronic pipettors which can be programmed with different volume settings and can be connected to a computer for data recording and analysis, this makes the process more efficient and user-friendly.
Why do we use pipetting?
Pipettes are used in laboratory settings to measure and transfer small volumes of liquid with high accuracy and precision. There are several reasons why pipettes are an important tool in the laboratory:
- Precision: Pipettes are precision instruments that can measure very small volumes of liquid with a high degree of accuracy. This is important when working with solutions that need to be precise in order to produce accurate results.
- Reproducibility: Pipettes can be used to transfer the same volume of liquid multiple times, which is important in experiments where reproducibility is a key factor.
- Efficiency: Pipettes can be used to measure and transfer multiple volumes of liquid at the same time, which increases the efficiency of the lab work.
- Versatility: Pipettes come in different sizes and designs, which allows them to be used in a variety of laboratory settings and applications.
- Safety: Pipettes can be used to handle hazardous or toxic liquids without the need for direct contact, which improves lab safety.
Pipetting is a skill that requires practice and precision. It is a crucial step in many laboratory procedures, such as preparing solutions, preparing samples for analysis, and running experiments. Therefore, pipettes are an important tool for scientists and technicians in many fields such as chemistry, biology, biochemistry, medical laboratories, food and environmental testing,and many others fields where small volume measurement is required.
Which pipetting method is commonly used and why?
The most commonly used pipetting method is referred to as “positive displacement” or “air displacement” method. This method is used in both manual and electronic pipettes and it is based on the principle that the amount of liquid displaced by a fixed volume of air is directly proportional to the volume of the liquid.
In this method, the user pulls a small volume of air into the pipette and immerses the tip into the liquid to be transferred. Then, the user pushes the thumb button or activates the electronic switch, this action releases the air from the pipette and pulls the liquid into the pipette. The user then verifies the volume of the liquid in the pipette, and then dispense the liquid into the desired container.
The positive displacement method is preferred for several reasons:
- High accuracy and precision: This method uses a fixed volume of air to displace a known volume of liquid, which makes it possible to measure small volumes with high accuracy and precision.
- Compatibility with viscous liquids: This method can be used with liquids of different viscosities, such as oils and syrups.
- Less evaporation: Positive displacement pipettes are sealed when aspirating and dispensing, which helps to reduce evaporation of volatile liquids.
- Lowest contamination: Pipettes that use the positive displacement method are less likely to contaminate the liquid being transferred, as the liquid is displaced by air, rather than being drawn up into the pipette by capillary action.
- Simplicity: Positive displacement method is relatively simple and easy to use, which makes it the most popular method for both manual and electronic pipettes.
However, it is worth mentioning that other method exist such as :
- Gravimetric method
- Vacuum method
- Measuring cylinder method
- Syringe method Each one of them has its own advantage and disadvantage. Depending on the type of the liquid, volume and the application, the laboratory and the researcher will choose the best suitable method.
What are lab pipettes called?
Lab pipettes are also known as laboratory pipettes, micropipettes, or just pipettes. They are precision instruments that are used to measure and transfer small volumes of liquid in a laboratory setting. They are commonly used in a variety of fields such as chemistry, biology, biochemistry, medical laboratories, food and environmental testing, and many others where small volume measurement is required.
There are different types of lab pipettes available, including single-channel pipettes and multi-channel pipettes. Single-channel pipettes are used to measure and transfer a single volume of liquid, while multi-channel pipettes can measure and transfer multiple volumes of liquid at the same time. Other types of pipettes include manual pipettes and electronic pipettes, which use a motor to control the movement of the liquid.
These pipettes come in different designs, sizes and volume ranges depending on the application, it can be classified as:
- Single channel pipettes ( fixed or adjustable volume)
- Multi-channel pipettes
- Electronic pipettes
- Repeating pipettes
- Serological pipettes
- Automatic pipettes
Each one of them has a specific use and application, and the laboratory will choose the most appropriate for their work.
The name “micropipettes” is sometimes used to refer to pipettes that can measure very small volumes of liquid, typically less than 1 milliliter. “Micro” meaning small, it is often used as a prefix in pipettes and other laboratory equipment that handle very small volumes.
What are the errors in pipetting?
Pipetting, like any measurement process, is subject to errors. These errors can come from a variety of sources, and can be classified into two main categories: systematic errors and random errors.
Systematic errors, also known as accuracy errors, are caused by systematic deviations from the true value of the volume being measured. They are often caused by factors such as the pipette not being properly calibrated, or not being used in accordance with the manufacturer’s specifications. Some examples of systematic errors in pipetting include:
- Incorrect calibration: If a pipette is not properly calibrated, it may measure a volume that is different from the true value.
- Use of worn or damaged parts: Worn or damaged parts can cause a pipette to measure volumes that are different from the true value.
- Human error: Pipetting is a skill that requires practice and precision, and human error can be a significant source of systematic errors.
Random errors, also known as precision errors, are caused by random variations in the measurement process. They are usually specified in terms of the standard deviation of a sample of repeated measurements of the same volume. Some examples of random errors in pipetting include:
- Variations in the position of the thumb or finger on the pipette: Small variations in the position of the thumb or finger can cause slight changes in the volume of liquid measured by the pipette.
- Variations in the viscosity of the liquid: Liquids with different viscosities may have slight variations in the volume that is displaced when pipetting.
- Variations in the temperature: Changes in the temperature of the liquid can affect the volume that is displaced when pipetting.
To minimize the errors in pipetting, it’s important to use proper techniques, calibration, and maintenance of the pipettes, and to be aware of the potential sources of error when performing a measurement. Also using electronic pipettes with automatic volume adjustment mechanism could also minimize the human errors. In general, the precision and accuracy of pipettes should be established by calibration, and the performance should be verified by regular testing.
Terms Related to pipetting
- Adjustment – Adjustment entails modifying the pipette such that the dispensed volume meets the requirements.
- Air Displacement Pipettes – are designed for use with aqueous solutions in general. A specific volume of air remains between the piston and the liquid in air displacement pipettes.
- Aspirate – to collect a sample.
- Blow-out – to totally empty the tip.
- Calibration check – Calibration check consists of comparing the dispensed volume to the desired volume.
- Deliver – to distribute the sample.
- Positive Displacement Pipettes – are used for liquids with a high viscosity or that are volatile. In positive displacement pipettes, the piston contacts the liquid directly.
How can I pipette viscous liquids?
Pipetting viscous liquids can be challenging, but there are a few techniques that you can use to make the process easier and more accurate.
- Use a positive displacement pipette: As I mentioned before, positive displacement pipettes are specially designed to handle viscous liquids. They work by physically trapping a specific volume of liquid within the pipette’s tip, which makes them well-suited for dispensing viscous liquids.
- Use a larger tip: Larger tips can help to reduce the resistance that viscous liquids exert on the pipette’s plunger, making it easier to dispense the liquid.
- Warm the liquid: If the viscous liquid is at room temperature or below, it can be helpful to warm it up to reduce its viscosity. It is important to do this safely by using a heating water bath.
- Use a pre-wetted tip: Before pipetting, you can wet the tip of your pipette with the liquid you are dispensing. This can help to reduce the surface tension of the liquid and make it easier to dispense.
- Use a mechanical dispenser: Some liquid handling systems have mechanical dispensers that can be useful to handle viscous liquids. The dispensers have a special design to help move the viscous liquid with less effort.
- Mix the liquid before pipetting: Mixing the liquid can also make it less viscous and therefore, easier to dispense. You can use a vortex mixer for this task.
It’s important to note that these techniques can help, but viscous liquids will still be more difficult to handle than liquids with low viscosity. Therefore, it is important to work carefully, pay attention to the settings of the instrument and the volume you are dispensing, and to calibrate your pipettes frequently.
How can I prevent liquid dropping out of the tip when pipetting volatile compounds?
Pipetting volatile compounds can be challenging because the liquid can easily evaporate, leading to drops forming on the tip of the pipette and dripping out. Here are a few techniques that you can use to prevent this from happening:
- Use a shorter tip: Using a shorter tip can help to reduce the distance between the liquid and the pipette’s plunger, which can help to prevent evaporation.
- Use a wider bore tip: A wider bore tip will decrease the time of liquid exposure to air, thus lessening the chances of evaporation.
- Use a positive displacement pipette: Positive displacement pipettes are specially designed to handle small volumes of liquid. They work by physically trapping a specific volume of liquid within the pipette’s tip, which makes them well-suited for dispensing volatile compounds.
- Cover the pipette tip: As you pipette, you can cover the tip of the pipette with a finger, or use a plastic or silicone tip cover, to prevent evaporation. However, it’s important to do it without touching the liquid.
- Keep your samples on a cooled environment: If possible, you can keep your samples in a cooled environment, like in an ice bath, to slow down evaporation.
- Pipette into sealed containers: Use sealed containers for your samples and pipette the volatile compound into them. This will minimize the exposure to air, reducing evaporation.
It’s important to note that volatile compounds will still be more difficult to handle than non-volatile liquids, so it is important to work carefully and pay close attention to the amount of liquid being dispensed. It is also essential to work in a well-ventilated area. Additionally, be sure to use a suitable technique for dispensing and storing the volatile compound, depending on the chemical properties of the compound.
Pipetting Technique Video
FAQ
What is meaning of pipetting?
Pipetting refers to the process of measuring and transferring small volumes of liquid using a device called a pipette. Pipettes are precision instruments that are used in a variety of laboratory settings, including chemistry, biology, and medicine. They are typically made of glass or plastic and come in different sizes and designs, depending on the type of work they will be used for.
There are several different types of pipettes, including single-channel and multi-channel pipettes. Single-channel pipettes are used to measure and transfer a single volume of liquid, while multi-channel pipettes can measure and transfer multiple volumes of liquid at the same time. Other types of pipettes include manual pipettes and electronic pipettes, which use a motor to control the movement of the liquid.
Pipetting is a crucial step in many laboratory procedures, such as preparing solutions, preparing samples for analysis, and running experiments. It is a skill that requires practice and precision, and it is important to use the correct technique to ensure accurate results.
There are two types of pipettes:
Volumetric pipettes : are calibrated to deliver a fixed volume of liquid.
Graduated pipettes : are calibrated to deliver variable volume.
Both type require different level of accuracy and precision
What is pipetting in a lab?
In a laboratory setting, pipetting is the process of measuring and transferring small volumes of liquid using a device called a pipette. Pipettes are precision instruments that are used in a variety of laboratory settings, such as chemistry, biology, and medicine. They come in different sizes and designs, depending on the type of work they will be used for.
When using a pipette in a lab, a scientist or technician will typically fill the pipette with the desired liquid by immersing the tip of the pipette into the liquid and drawing the liquid up into the pipette. They may then adjust the volume of the liquid in the pipette by adjusting the position of a thumb or finger on the pipette or by using a digital or manual adjustment mechanism. Once the correct volume is reached, the liquid can be dispensed into another container or onto a surface.
Pipetting is a crucial step in many laboratory procedures, such as preparing solutions, preparing samples for analysis, and running experiments. It is a skill that requires practice and precision, and it is important to use the correct technique to ensure accurate results.
The protocols of pipetting vary on the laboratory , type of the liquid and the volume to be transferred. It also depends on the type of the pipette, manual or electronic .
Proper technique and use of pipettes, such as using the correct tip size for the desired volume, properly calibrating the pipette, and keeping the pipette clean and in good working condition, are critical to obtaining accurate and reliable results in a laboratory setting.
What is precision in pipetting?
Precision in pipetting refers to the ability of a pipette to consistently and accurately measure and transfer a specific volume of liquid. A pipette is considered to be precise if, when measuring and transferring the same volume of liquid multiple times, the volumes obtained are very similar to one another.
Pipettes are precision instruments and have a small measurement uncertainty, which is usually specified in terms of the standard deviation of a sample of repeated measurements of the same volume, called the precision error. The precision error of a pipette is usually measured and specified by the manufacturer, or it can be determined by lab calibration.
Precision is an important aspect of pipetting because accurate results depend on precise measurement of the liquid volumes. For example, in experiments where reproducibility is a key factor, precise pipetting is necessary to ensure that the same volume of liquid is used each time. In addition, in analytical chemistry, where very small volumes of liquid are often used, precise pipetting is necessary to ensure accurate results.
However, precision alone is not enough to guarantee the accuracy of the measurement. Pipettes, like any measurement instruments, also have an inherent systematic error, called the accuracy error.
This type of error is usually determined by using a reference standard that is traceable to a national or international standard.
To guarantee accurate measurement, both precision and accuracy should be considered and controlled. This can be done through regular calibration, proper maintenance and use of pipettes, and using the correct technique when pipetting.
What is the difference between air displacement and positive displacement pipettes?
Air displacement pipettes and positive displacement pipettes are two types of laboratory pipettes that are used to measure and dispense small volumes of liquid.
Air displacement pipettes, also known as air-jet or jet pipettes, work by creating a stream of air that is used to aspirate liquid into the pipette. The user sets the volume to be dispensed by adjusting the position of the pipette’s tip within the pipette’s barrel. The user then pushes the pipette’s plunger to dispense the liquid. The volume of liquid that is dispensed is determined by the position of the plunger and the size of the pipette’s tip.
Positive displacement pipettes, on the other hand, work by physically trapping a specific volume of liquid within the pipette’s tip. The user sets the volume to be dispensed by adjusting the position of the pipette’s plunger, and then pushes the plunger to dispense the liquid. The volume of liquid that is dispensed is determined by the volume of the pipette’s tip and the position of the plunger. Positive displacement pipettes are commonly used for dispensing viscous liquids, such as oils and suspensions.
In general, positive displacement pipettes are more accurate and precise than air displacement pipettes, but they are also more expensive and can be more difficult to use. For this reason, air displacement pipettes are often used for routine tasks that do not require high levels of accuracy and precision, while positive displacement pipettes are used for more critical applications.
While air displacement pipettes use air to measure the volume, positive displacement pipettes do not use air. Instead, they use the shape of the tip, the movement of the piston, and the properties of the liquid to dispense a specific volume.
How accurately can I pipette warm or cold liquids.
The accuracy of pipetting can be affected by the temperature of the liquid being dispensed. Pipetting warm or cold liquids can cause the liquid to expand or contract, which can affect the volume of liquid that is dispensed.
When dispensing warm liquids, the liquid will expand and the volume dispensed will be larger than intended, leading to over-dispensing. This can be corrected by adjusting the volume setting on the pipette, or by allowing the liquid to cool before dispensing.
On the other hand, when dispensing cold liquids, the liquid will contract and the volume dispensed will be smaller than intended, leading to under-dispensing. This can be corrected by adjusting the volume setting on the pipette, or by allowing the liquid to warm up before dispensing.
To achieve the best accuracy while dispensing warm or cold liquids, it is important to use a calibrated pipette and to use the appropriate technique to adjust for the effects of temperature. Ideally, liquids should be brought to a room temperature before dispensing and during the dispensing process the pipette should be kept at the same temperature, this way the temperature changes will have a minimal effect on volume.
Additionally, positive displacement pipettes are less affected by the temperature change, as they work by trapping the liquid inside the tip and not relying on the air displacement. However, it is always recommended to check the accuracy of the pipette before and after dispensing, to ensure that the readings are accurate.