Do you think phosphorus is important? Does this depend on whether you want DNA, cell membranes, text, or bones within your body? It’s a good bet that the likelihood is that yes!
Phosphorus is a vital food element in living beings. It is a vital component of nucleic acid molecules, such as the DNA start DNA, and of the phospholipids which create the cell membranes. In the form of calcium phosphate, it also forms the supportive bones.
In the natural world, phosphorus can be the most limiting nutrient, or in terms of the most abundant nutrient and limits development. This is especially the case in freshwater and aquatic ecosystems.
- Phosphorus is a vital nutrient found in macromolecules of human and other living things, such as DNAstart text in text D NA, the end text.
- The process of phosphorus is very slow. The majority of phosphorus found in nature is as the phosphate ion–(PO4)^3
- Phosphorus is usually the most limiting nutrients, or the nutrient that is most scarce , which hinders growth in ecosystems with aquatic life.
- In the event that nitrogen and phosphorus from fertilizers are transported by the runoff of lakes and oceans and oceans, they may cause an overgrowth of algae. The algae can reduce oxygen levels in the water and cause an area of deadness.
Definition of Phosphorus Cycle
Phosphorus cycling is a biogeochemical process which is characterized by the flow of phosphorus across the hydrosphere, the lithosphere, and biosphere.
- Phosphorus is a vital component for all living creatures since it is the basic base for nucleotides and serves as a mineral to aid in development.
- Contrary to other biogeochemical processes, the atmosphere does not function as reservoirs for phosphorus during the phosphorus cycle because the majority of the phosphorus-containing substances that are involved within the process are the form of solids.
- The phosphorus cycle comprises of a variety of biological, chemical and microbiological processes, which all occur over a prolonged time.
- The cycle is comprised of processes such as weathering, which take many years for completion. Therefore the phosphorus cycle can be thought to be one of the most slow biogeochemical cycles.
- Phosphorus is one of the most rare elements in nature. This makes it among the most limiting agents.
- Phosphorus compounds travel through the biosphere, oceanic and terrestrial, to keep a steady balance in their concentration.
- But, the majority of compound phosphorus is found in the lithosphere where they are found in sedimentary deposits and rocks.
- Since phosphorus is a highly reactive chemical It is usually present in a combination condition along with other elements. Therefore, acid-producing microorganisms are essential to make soluble phosphate from insoluble elements.
- The amount of phosphorus found in various reservoirs fluctuates over time, with the phosphorus in the soil being released to oceans , as well as the phosphorus found in the ocean, leading to sediments.
- The phosphorus cycle can be described as closed with the transfer of phosphorus between the soil and biosphere is more extensive than the losses and gains across the entire system.
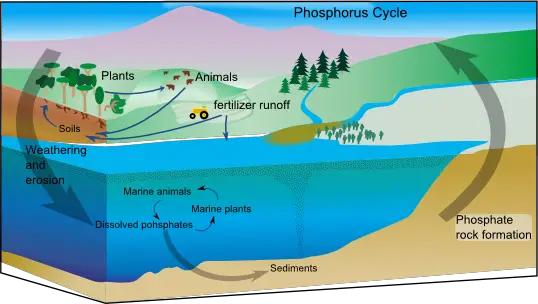
Phosphorus Cycle Steps
The mechanism that drives the phosphorus cycle isn’t as well understood in comparison to other cycles that are biogeochemical. It is however known that the phosphorus cycle is a long process that consists of these steps
1. Weathering
Rocks are among the major reservoirs and sources of phosphorus. They remain mixed along with the other components. The phosphorus that is in soil is then released into the soil via rain, or through the production of acid by various microorganisms. Different microorganisms like Actinomycetes, Pseudomonas, Bacillus, Aspergillus, Penicillium, etc. participate in solubilization process of phosphorus in soils in order to make it accessible to animals and plants. Additionally, rain triggers the solubilization of phosphorus inorganic to allow it to be transported to oceans. Natural processes such as eruptions of volcanoes and asteroid activity can also assist with the release of phosphorus into soil.
2. Phosphorus intake by plants
The phosphorus that is present in the soil is utilized by various living creatures like microorganisms and plants. It is evident that the amount of phosphorus that plants can absorb in the soil is smaller, which is why fertilizers containing phosphorus should be used to enhance the growth of plants and improve soil fertility. The marine plants absorb phosphorus from lower layers of sediments in the water. However, certain phosphorus salts may not dissolve correctly in water, which means that the amount of phosphorus available in water could be restricted. Plants could either absorb the phosphorus from soil directly or be released to plants by microorganisms in symbiosis.
3. Movement of phosphorus in the food chain
The phosphorus that is absorbed by plants is used to make diverse organic compounds in producers. Organic compounds are then moved across the food chain, as consumers consume producers, leading to the movement of phosphorus one kind of life form to another. Organic phosphorus undergoes modifications as it travels through consumers. The phosphorus that is transferred to consumers is used in the production of biomolecules, such as the nucleotides, and connective tissue such as bones.
4. Return of phosphorus to the ecosystem
The phosphorus found in living creatures can be returned to the reservoir within the lithosphere through the process of decomposing microorganisms on dead animal and plant. This is where all organic forms of the phosphorus get transformed into inorganic counterparts by this process called mineralization.
Many saprophytic microorganisms that decompose such as bacteria and fungi participate in this stage to control the amount of phosphorus within the ecosystem. The phosphorus present in soil can also be transferred to oceans in rain or through the flowing off of the soil into waters bodies. The ocean’s phosphorus gets deposition and forms layers of sediments which will eventually lead to the formation of rocks , and the process continues.
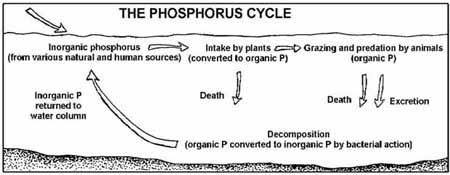
Phosphorus Cycle Examples
In addition to the main process of phosphorus, there exist other parallel systems involved in the transportation of phosphorus across various reservoirs, in order to keep the balance. There are a variety of reservoirs within diverse ecosystems, each capable of absorbing or release phosphorus transport one form to the next.
1. Lithosphere
The largest reservoir of phosphorus on Earth is in the lithosphere. It is dominated by freshwater and oceanic sediments. The first source of the phosphorus found on Earth is found through chemicals and weathering, as well as the soil development processes. These substances are formed through natural processes, such as earthquakes and volcanoes, or enhance the phosphorus content of the soil.
In addition, during the cycle process, the phosphorus flows back to the soil as the soil runoff containing phosphorus reaches the oceans. The soil particles are layered of sediments under the water that eventually form minerals-rich rocks.
2. Biosphere
The terrestrial biosphere as well as the ocean’s phosphorus reservoir contribute to the process of phosphorus. A majority of phosphorus not accessible to living things since it is in a non-soluble form. The biosphere serves as a reservoir where surplus phosphorus may be stored. Living organisms absorb the phosphorus and form various biomolecules, which are vital to their development and function.
The microorganisms that live in the biosphere play an important role in that they assist in the solubilization of inorganic phosphorus and make it accessible to the producers. The biosphere is an intermediary in the process of recycling the phosphorus back into the lithosphere.
Phosphorus Cycle Significance
- It is vital in balancing the amount of phosphorus at the Earth’s surface to provide a comfortable world.
- Phosphorus is an essential elements that are required by all living things and its movement through various systems aids in identify the different biological elements and the factors that affect them.
- The phosphorus cycle is linked to that of the other element and compound such as sulfur and nitrogen as it is present in a combination form along with other elements found in the natural world.
- The phosphorus cycle facilitates the flow of energy it’s form ATP across the chain. The phosphorus compounds transport chemical energy that is absorbed by producers to consumers and then decomposers.
- The mineralization or decomposition of phosphorus is just one of the steps that are part of the natural system of waste disposal.
- The Phosphorus cycle helps increase the amount of phosphorus in soil to support plant growth as well as soil fertility.
- Understanding the mechanisms of the phosphorus cycle is helpful to comprehend the physiology of various microorganisms with the cycle.
Human impacts on the Phosphorus Cycle
- The use of synthetic fertilizers on soil can affect the levels of phosphorus within the soil. This influences the overall cycle of phosphorus.
- The most significant change anthropogenic causes in the cycle of phosphorus massive transfer of the massive and unobtainable reserve pool to the biologically accessible forms that are on the land.
- The mining of phosphate-rich rock also affects the amount of phosphorus available in the various ecosystems.
- The rise in the amount of phosphorus in oceans as a result soil runoff causes an increased development of algae blooms. This reduces the oxygen supply in the ocean.
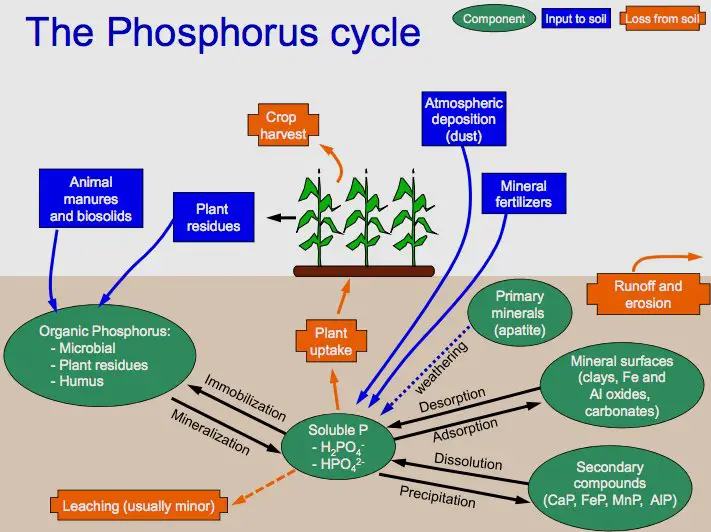
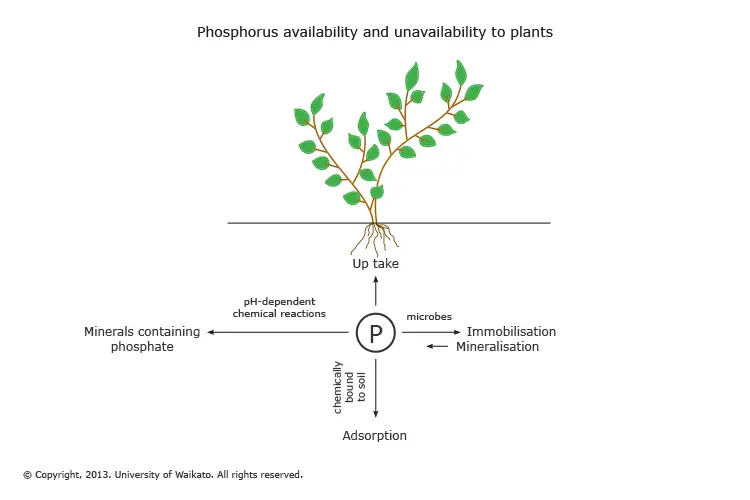
Most phosphorus is unavailable to plants
Because the majority of our phosphorus is stored in rocks and sediments the phosphorus isn’t available to plants to consume. A large portion of the phosphorus found in soils isn’t available to plants.
The amount of phosphorus available in soil for plants is dependent on various reversible pathways
- Bacteria: Bacteria transform the phosphate that is available to plants into organic forms, which can then be unavailable to plants. While some bacteria are able to make phosphate accessible through mineralisation, their contribution to this is comparatively small.
- Adsorption: Inorganic (and readily available) and available phosphorus is physically bound (adsorbed) into soil particles rendering it inaccessible to plants. Desorption is the process of releasing the phosphorus adsorbed out of its bound form to soil solutions.
- pH: The inorganic phosphorus compounds require soluble conditions in order to be absorbed by plants. This is dependent upon the pH (pH) that the soil has. If the soil is less then pH 4 and more than pH 8.8, the phosphorus begins to get entangled with other substances which makes it unavailable to plants.
The majority of plants require more phosphorus than what is present in soil in order to thrive. Furthermore, crops are typically removed after harvest, leaving no decaying vegetation in place to take over the phosphorus. Thus farmers replenish the phosphorus pool by using fertilisers or effluents to replace the phosphorus used from plants.
What are Eutrophication and dead zones?
The majority of fertilizers used in agriculture, and on gardens and lawns contain nitrogen and phosphorus. These could be transported into aquatic ecosystems via runoff from the surface. Runoff fertilizers may result in an excessive growth of algae, or other microbes prior to being restricted by nitrogen or the phosphorus. This is known as eutrophication. In some instances the phosphorus element, not nitrogen is believed to be the primary driving force behind eutrophication.^2
How can eutrophication be harmful? Certain algae cause water to smell or taste bad, or create harmful compounds.^2 Furthermore when all the algae die and get destroyed by microbes and microbes, massive quantities of oxygen are consumed because their bodies are destroyed. This increase in oxygen use can dramatically decrease levels of oxygen in the water. It could also cause death due to hypoxia, or a lack of oxygen for other aquatic organisms like finfish and shellfish.
Oceans and lakes that have been reduced in oxygen levels due to an influx of nutrients are known as dead zones. Dead zones has been increasing for a number of years, and over 400 of them existed in the year 2008. One of the dead zones is located off along the coastline of the United States in the Gulf of Mexico. Fertilizer runoff from Mississippi River Basin created a dead zone that covers more than 8463 square miles. As you will see in the graphic that follows, dead zones can be located in areas with high population density and industrialization across the globe.
How can eutrophication can be diminished or prevented? Fertilizers, phosphorus-containing detergents, and improperly disposed of sewage can all be sources of nitrogen and phosphorus that drive eutrophication. Using less fertilizer, eliminating phosphorus-containing detergents, and ensuring that sewage does not enter waterways–e.g., from a leaky septic system–are all ways that individuals, companies, and governments can help reduce eutrophication.
What is the role of phosphorus in animals and plants?
Phosphorus is a vital nutritional element that is required by both animals and plants. It plays a crucial role in cell growth and is an essential element in the synthesis of molecules that conserve energy, like ATP (adenosine triphosphate) DNA, DNA, as well as the lipids (fats and oil). A lack of phosphorus in soil can lead to lower yield for crops.