What are Neutrophils?
- Neutrophils, also known as neutrocytes, heterophils, or polymorphonuclear leukocytes, play a crucial role in our immune system as the most abundant type of granulocytes. In humans, they make up a significant portion, ranging from 40% to 70%, of all white blood cells.These remarkable cells are integral to the innate immune system, and their functions can vary across different animal species.
- The formation of neutrophils begins in the bone marrow, where stem cells give rise to these white blood cells. Once differentiated, they can be classified into two subpopulations: neutrophil-killers and neutrophil-cagers. Neutrophils have a relatively short lifespan, ranging from 5 to 135 hours, and they exhibit exceptional mobility, allowing them to infiltrate tissues inaccessible to other cells or molecules. Neutrophils are further categorized as segmented neutrophils and banded neutrophils, often referred to as bands. Alongside basophils and eosinophils, they belong to the polymorphonuclear cells family (PMNs).
- The name “neutrophil” stems from its staining characteristics observed in hematoxylin and eosin (H&E) histological or cytological preparations. While basophilic white blood cells stain dark blue and eosinophilic white blood cells stain bright red, neutrophils appear as a neutral pink color. Typically, neutrophils possess a nucleus divided into 2–5 lobes.
- Neutrophils primarily function as phagocytes and normally reside in the bloodstream. During the initial phase of inflammation, particularly in response to bacterial infections, environmental exposure, or certain cancers, neutrophils swiftly migrate towards the inflamed site, acting as first responders among the inflammatory cells. They navigate through blood vessels and interstitial spaces, guided by chemical signals such as interleukin-8 (IL-8), C5a, fMLP, leukotriene B4, and H2O2, in a process known as chemotaxis. Pus, characterized by its whitish or yellowish appearance, predominantly consists of these infiltrating neutrophils.
- Within minutes of trauma or injury, neutrophils are recruited to the affected area, serving as a hallmark of acute inflammation. However, certain infections may prove challenging for neutrophils to resolve independently, as some pathogens are resistant to digestion. In such cases, the assistance of other types of immune cells becomes necessary to overcome the infection successfully. Nonetheless, neutrophils remain at the forefront of our immune response, forming an essential part of our body’s defense against pathogens and contributing to our overall health and well-being.
Neutrophils Definition
Neutrophils are a type of white blood cell that plays a crucial role in the immune system’s defense against infections. They are highly mobile and act as phagocytes, engulfing and destroying bacteria and other harmful substances. Neutrophils are characterized by their multi-lobed nuclei and stainable cytoplasmic granules. They are part of the innate immune system and are among the most abundant white blood cells in the bloodstream.
Features of Neutrophils
Neutrophils possess distinctive features that contribute to their essential role in the immune system’s defense against microbial infections. These features include:
- Phagocytosis: Neutrophils are highly efficient phagocytes, capable of engulfing and destroying invading microorganisms. They recognize pathogens through pattern recognition receptors (PRRs) on their cell surface, triggering the process of phagocytosis. Once a microorganism is engulfed, it is enclosed within a phagosome, which then fuses with lysosomes containing antimicrobial peptides, enzymes, and reactive oxygen intermediates. This fusion results in the degradation and elimination of the microorganism.
- Degranulation: Neutrophils store potent antimicrobial substances, including enzymes and antimicrobial peptides, within cytoplasmic granules. Upon activation, these granules are released into the surrounding environment, enabling the neutrophils to directly kill microorganisms extracellularly. The antimicrobial substances disrupt the integrity of microbial membranes and neutralize their virulence factors, contributing to the eradication of the infection.
- Neutrophil Extracellular Traps (NETs): Neutrophils have a unique ability to release their DNA, along with antimicrobial proteins, to form neutrophil extracellular traps (NETs). NETs are web-like structures that can ensnare and kill microorganisms. This process, known as NETosis, helps to immobilize pathogens, preventing their spread and facilitating their elimination by other immune cells.
- Rapid Response: Neutrophils are among the first immune cells to migrate towards the site of inflammation or infection. They can quickly move from the bloodstream into tissues, guided by chemotactic signals released by the site of injury or infection. This rapid response ensures that neutrophils reach the site early, allowing them to initiate the immune defense promptly.
- Short Lifespan: Neutrophils have a relatively short lifespan, ranging from a few hours to a few days. This limited lifespan is due to their high metabolic activity and the potential for damage caused by their antimicrobial mechanisms. The short lifespan also ensures that neutrophils do not accumulate excessively, preventing unwanted tissue damage.
- Mobility: Neutrophils exhibit high motility, enabling them to move freely within blood vessels and extravasate into tissues. They can squeeze through narrow spaces, migrate along chemical gradients, and navigate complex tissue environments to reach the site of infection efficiently.
The combination of these features allows neutrophils to mount a rapid and potent defense against microbial invaders. Their ability to phagocytose, degranulate, release NETs, and their remarkable mobility make them critical players in the innate immune response, safeguarding the body against infectious threats.
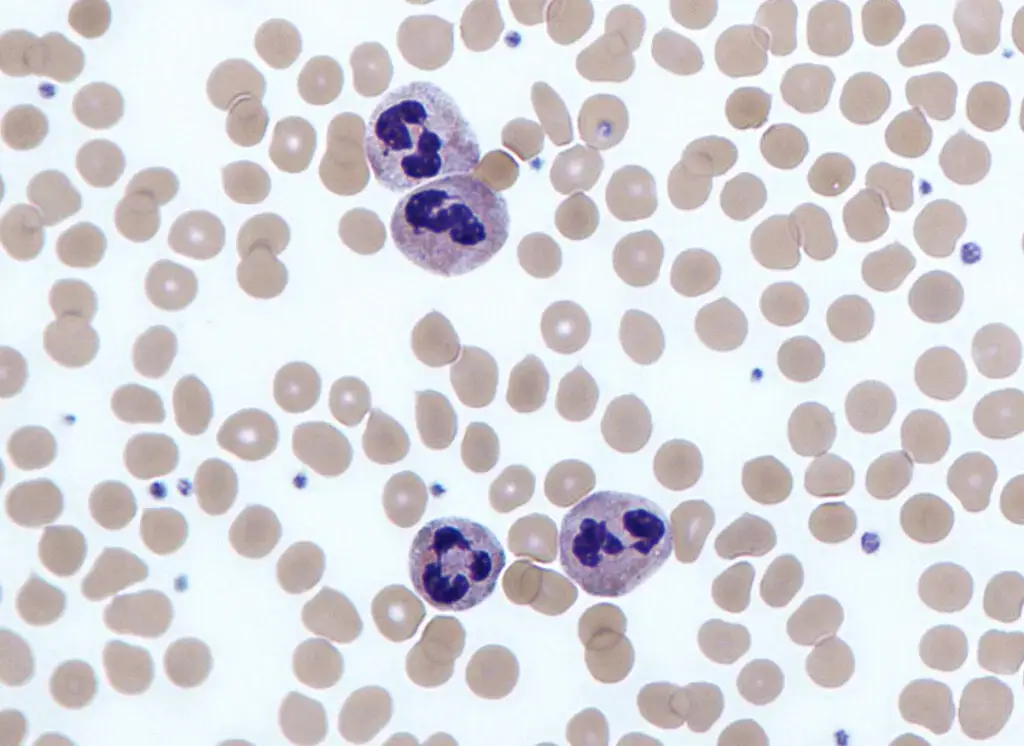
Neutrophils test/ Absolute Neutrophil Count
The Absolute Neutrophil Count (ANC) is a vital test that allows healthcare professionals to determine the number of neutrophils and other granulocytes, collectively known as polymorphonuclear cells, present in a blood sample. This test provides valuable insights into the overall white blood cell count, encompassing both mature and immature neutrophils.
The neutrophil blood count is further divided into two categories: segmented or mature neutrophils and immature neutrophils, also known as bands. By analyzing these specific components, healthcare providers can identify any abnormalities related to an increase or decrease in the number of neutrophils.
The abnormal count of neutrophils is often associated with various medical conditions, underscoring the significance of this test as an essential component of laboratory examinations for many diseases. It serves as a diagnostic tool to detect and monitor the presence of different organisms in the bloodstream, as well as assess the proper functioning of the immune system.
To calculate the Absolute Neutrophil Count (ANC), a specific formula is employed:
ANC = Absolute mature neutrophils + Absolute immature neutrophils
By utilizing this formula, healthcare professionals can obtain an accurate measurement of the neutrophil count, providing valuable information about the patient’s immune response.
The ANC test is typically performed as part of a complete blood count, which comprehensively measures the count of various blood cells. This comprehensive evaluation aids in assessing overall health and detecting any potential abnormalities or irregularities in the blood composition.
In summary, the Absolute Neutrophil Count (ANC) test plays a crucial role in diagnosing and monitoring a range of medical conditions. By accurately quantifying the neutrophil count, healthcare professionals can gain valuable insights into the functioning of the immune system and detect any deviations from the normal range, enabling prompt medical intervention and treatment.
Neutrophils normal range
The number of neutrophils present in the blood can vary from person to person, influenced by factors such as age and the environment. However, the following ranges are generally considered to be within the normal range for neutrophil count.
When assessing the absolute cell count, the normal range for adults is typically as follows:
- Absolute Neutrophil Count (ANC): 1500-8000 cells/mm3
- Mature/Segmented Neutrophils: 2500-6000 cells/mm3
- Immature Neutrophils: 0-500 cells/mm3
Alternatively, the percentage of neutrophils in relation to the total white blood cell (WBC) count can also indicate normal ranges. For adults, these ranges are typically as follows:
- ANC as a percentage of WBC: 40-45%
- Mature/Segmented Neutrophils as a percentage of WBC: 40-60%
- Immature Neutrophils as a percentage of WBC: 0-5%
It’s important to note that these ranges serve as general guidelines and may vary slightly depending on the specific laboratory and reference values used. Additionally, certain factors such as underlying medical conditions or individual variations can influence the normal range for neutrophil counts in specific cases. It is always advisable to consult with a healthcare professional for a comprehensive evaluation of your blood test results and to interpret them within the context of your overall health.
Factors Affecting Neutrophil Count
Various factors can affect the neutrophil count in the blood. These include:
- Age: The neutrophil count varies with age. Newborns have a higher neutrophil count than adults, with a normal range of 10000-30000 cells/mm3. Children between 2-6 years of age have a normal range of 2000-8000 cells/mm3.
- Health conditions: Certain health conditions can cause a deviation from the normal range of neutrophil count. Infections, inflammation, and autoimmune disorders can increase the neutrophil count, while bone marrow disorders and chemotherapy can decrease the neutrophil count.
- Medications: Certain medications like corticosteroids, lithium, and heparin can decrease the neutrophil count.
- Genetics: Some people may have a genetic predisposition to low neutrophil count, known as neutropenia.
- Environment: Exposure to radiation, chemicals, and toxins in the environment can affect the neutrophil count.
- Lifestyle: Smoking, excessive alcohol consumption, and a poor diet can also affect the neutrophil count.
Measuring the neutrophil count is a crucial aspect of assessing the immune system’s health. The normal range of neutrophil count varies with age and is expressed in two ways – absolute neutrophil count and percentage of the WBC count. Various factors like age, health conditions, medications, genetics, environment, and lifestyle can affect the neutrophil count. If you have concerns about your neutrophil count, consult a healthcare professional for an accurate diagnosis and appropriate treatment.
Neutrophils low Level
- When the neutrophil count is below 1500 cells/mm3 of blood volume, the neutrophil count is regarded to be low.
- This disorder is also known as neutropenia. Mild neutropenia is characterised by neutrophil counts between 1000 and 1500 cells per millimetre. The range between 500 and 100 cells/mm3 is known as mild neutropenia. When neutrophil counts fall below 500 cells/mm3, severe neutropenia is seen.
- It is common to detect a low neutrophil count after taking medicine, but it can also be caused by other factors or illnesses.
Causes of Low Neutrophil Level (Neutropenia):
- Medications: The intake of certain medications, especially those used in chemotherapy, can lead to a low neutrophil count.
- Suppressed Immune System: Underlying diseases that suppress the immune system, such as AIDS, tuberculosis, and hepatitis, can cause a decrease in neutrophil levels.
- Cancer and Bone Marrow Diseases: Conditions like cancer and bone marrow disorders can interfere with the production of neutrophils, resulting in low counts.
- Deficiencies: Deficiencies of vitamin B12 and other essential minerals can contribute to neutropenia.
- Autoimmune Diseases: Autoimmune conditions like Crohn’s disease, lupus, and rheumatoid arthritis can cause a decrease in neutrophil count as a result of immune system dysfunction.
Neutrophil high Level
- When the number of neutrophils exceeds 8,000 cells/mm3, the level is regarded to be high.
- This disorder is known as neutrophilia. Neutrophilia can range from modest, infrequent neutrophilia to neutrophil leukocytosis, a more serious disorder.
- Due to the fact that neutrophils are part of the immune system, a rise in neutrophil count is primarily caused by a bacterial infection, but other causes may also contribute.
Causes of High Neutrophil Level (Neutrophilia):
- Bacterial Infection: The most common cause of neutrophilia is a bacterial infection, particularly pyogenic infections that lead to inflammation.
- Inflammation: Neutrophil count can increase during episodes of inflammation, often seen after conditions like heart attacks or burns.
- Hormonal Imbalances: Elevated levels of cortisol and adrenaline hormones can cause an increase in neutrophil count. Certain medications, such as prednisone, can also contribute to neutrophilia.
- Malignancy: Neutrophilia can occur as a result of malignancies, including leukemia, where abnormal proliferation of white blood cells, including neutrophils, leads to an increased count.
- Surgical Procedures: Certain surgical procedures, such as splenectomy (removal of the spleen) or appendicitis, can trigger an increase in neutrophil count as a response to tissue damage or infection.
Life span of Neutrophil
- The life span of neutrophils, the most abundant type of white blood cells, varies depending on their activation state and location within the body. In their inactivated state circulating in the bloodstream, the average lifespan of human neutrophils has been reported to range from 5 to 135 hours, equivalent to approximately 5 days to 15 hours.
- Upon activation, neutrophils undergo a series of dynamic processes. They first marginate, positioning themselves adjacent to the endothelium of blood vessels. Subsequently, they undergo selectin-dependent capture, followed by integrin-dependent adhesion in most cases. These processes allow the neutrophils to firmly adhere to the vessel walls. Once firmly attached, they migrate into tissues, where they can actively combat invading pathogens. In the tissue environment, neutrophils have a relatively shorter lifespan, typically surviving for 1 to 2 days.
- Interestingly, neutrophils can also be released into the bloodstream from a splenic reserve following myocardial infarction, providing additional support to the immune response in certain situations.
- Compared to the longer-lived monocyte/macrophage phagocytes, neutrophils have a shorter lifespan. This disparity in longevity is thought to be an evolutionary adaptation. Neutrophils, with their shorter lifespan, are strategically positioned to be the first responders to pathogenic encounters. This rapid response ensures that pathogens are more likely to be encountered and engulfed by neutrophils before they can establish a more substantial infection. By limiting the time pathogens spend outside of host cells, the risk of their propagation is minimized. Additionally, neutrophils produce antimicrobial products that can also cause damage to host tissues. Therefore, their shorter lifespan serves to limit potential damage to the host during inflammatory processes.
- Once neutrophils have completed their task of phagocytosing pathogens, they are typically removed from the body by macrophages. The process of removal involves specific molecules on the surface of neutrophils, such as PECAM-1 and phosphatidylserine, which are recognized by macrophages.
- Understanding the life span of neutrophils provides valuable insights into the dynamics of the immune response and highlights their critical role in combating infections while minimizing damage to the host.
Structure of Neutrophils
- Neutrophils, a type of white blood cell, possess a distinct structure that enables them to carry out their crucial role in the immune response. These cells typically exhibit a circular shape, measuring between 12 and 15 µm in diameter, although in humans, the average size is around 8 µm. Once activated, neutrophils undergo a remarkable transformation, adopting an amoeboid shape that allows them to extend pseudopodia, or false feet, to effectively engage and attack invading pathogens.
- One prominent feature of neutrophils is their multi-lobed nucleus, which sets them apart from other types of white blood cells. The nucleus typically comprises three to five lobes connected by a slender strand of genetic material. It is worth noting that in younger neutrophils, a nucleolus can be observed within the nucleus, but as these cells mature, the nucleolus disappears—a phenomenon observed in only a limited number of other nucleated cells.
- The cytoplasm of neutrophils presents numerous purple-colored granules, known as azurophilic or primary granules, which possess microbicidal activity. These granules play a crucial role in neutralizing and eliminating pathogens. Additionally, secondary granules are also present within the cytoplasm, housing enzymes such as lysozyme and collagenase. Although other organelles, including mitochondria and the Golgi complex, appear in limited quantities, the rough endoplasmic reticulum is entirely absent from neutrophils.
- When adhered to a surface, neutrophil granulocytes exhibit an average diameter of 12-15 µm as observed in peripheral blood smears. In suspension, human neutrophils possess a slightly smaller average diameter of 8.85 µm. Neutrophils, along with eosinophils and basophils, constitute the class of polymorphonuclear cells, owing to the multilobulated shape of their nuclei. The distinct lobes of the nucleus are connected by chromatin. Notably, during neutrophil maturation, the nucleolus diminishes—a characteristic shared by only a few other nucleated cell types. Furthermore, in approximately 17% of female human neutrophils, a drumstick-shaped appendage can be observed, which contains the inactivated X chromosome.
- Within the cytoplasm, neutrophils possess a small Golgi apparatus, sparse mitochondria and ribosomes, and an absence of the rough endoplasmic reticulum. Approximately 200 granules can be found in the cytoplasm, with about one-third classified as azurophilic granules. As neutrophils mature, their nuclei display increasing segmentation, with a normal neutrophil featuring 3-5 segments. Hypersegmentation, characterized by the presence of 5 or more segments in most or all neutrophils, is abnormal and may indicate certain disorders, notably vitamin B12 deficiency.
- In terms of their abundance, neutrophils are the most prevalent white blood cells in humans, with approximately 10^11 being produced daily. They account for roughly 50-70% of all white blood cells, or leukocytes. Normal ranges for neutrophil counts can vary across laboratories, but a standard range is typically considered to be between 2.5-7.5 × 10^9/L. It’s important to note that individuals of African and Middle Eastern descent may have lower counts, which still fall within the normal range. Reports often categorize neutrophils into segmented neutrophils and bands.
- When circulating in the bloodstream in an inactive state, neutrophils assume a spherical shape. However, upon activation, they undergo a remarkable metamorphosis, becoming more amorphous or amoeba-like. This shape change allows neutrophils to extend pseudopods, enabling them to actively seek out and engage antigens.
- Studies have shed light on the influence of sugar intake on neutrophil function. Research conducted by Sanchez et al. in 1973 revealed that the ability of neutrophils to engulf bacteria was diminished upon ingestion of simple sugars such as glucose, fructose, sucrose, honey, and orange juice. Conversely, fasting was found to strengthen the neutrophils’ phagocytic capacity to engulf bacteria. These findings suggested that the function, rather than the quantity, of phagocytes in bacterial engulfment was altered by sugar intake. In 2007, researchers at the Whitehead Institute of Biomedical Research discovered that neutrophils exhibited a preference for certain types of sugars on microbial surfaces. They demonstrated a preferential engulfment and elimination of beta-1,6-glucan targets compared to beta-1,3-glucan targets.
- The structure of neutrophils, with their unique nuclear morphology, granule composition, and shape-changing abilities, underscores their essential role in the immune response. These versatile cells exemplify the intricate mechanisms employed by our immune system to combat pathogens and maintain overall health.
Functions of Neutrophils
- Phagocytosis: Neutrophils are adept at engulfing and devouring foreign microorganisms, particularly bacteria. They utilize their phagocytic ability to trap bacteria within phagosomes.
- Destruction of Invaders: Once bacteria are trapped within phagosomes, neutrophils unleash a barrage of potent enzymes to degrade and destroy them. This process ensures the elimination of the invading pathogens.
- Secretion of Antimicrobial Proteins: Neutrophils secrete a range of proteins with antimicrobial effects. These proteins aid in neutralizing and eliminating pathogens, further enhancing the immune response.
- Tissue Remodeling: Apart from their antimicrobial role, neutrophils also contribute to tissue remodeling. They release factors that facilitate the repair and healing of damaged tissues.
- Short Lifespan: Neutrophils have a relatively short lifespan, usually ranging from a few hours to a few days. During the process of eliminating foreign invaders, neutrophils undergo self-destruction. This controlled cell death, known as apoptosis, helps prevent excessive inflammation and tissue damage.
- Continuous Production: To maintain a steady supply, new neutrophils are continuously generated in the bone marrow. This ensures that the immune system has a constant pool of these vital cells ready to respond to any infectious threat.
- Carrier Neutrophils: Within the neutrophil population, there are specialized subsets, such as carrier neutrophils. These cells have a specific transport function, delivering foreign particles to target sites. Their role is to facilitate the actions of other neutrophil subtypes, like killer neutrophils, in effectively eliminating threats.
- Integral Immune Defense: Neutrophils play a crucial role in the body’s innate immune defense system. They are among the first cells to arrive at the site of infection, acting as frontline defenders against invading pathogens.
FAQ
What are neutrophils?
Neutrophils are a type of white blood cell that plays a crucial role in the immune system. They are the first responders to fight off infections and foreign substances that enter our body.
What is the normal range of neutrophil count?
The normal range of neutrophil count varies with age and is expressed in two ways – absolute neutrophil count (ANC) and percentage of the white blood cell (WBC) count. In adults, the normal range of ANC count is 1500-8000 cells/mm3, and the normal range of mature/ segmented neutrophils is 2500-6000 cells/mm3. The normal range of immature neutrophils is 0-500 cells/mm3. The normal range of ANC count in adults is 40-45%, the normal range of mature/ segmented neutrophils is 40-60%, and the normal range of immature neutrophils is 0-5%.
What are the functions of neutrophils?
Neutrophils play a crucial role in the immune system, and their primary function is to defend the body against infections and foreign substances. They do this by engulfing and digesting invading bacteria, fungi, and viruses.
What is neutropenia?
Neutropenia is a medical condition characterized by low neutrophil count in the blood. It can be caused by a variety of factors, including medications, chemotherapy, infections, and bone marrow disorders.
What is neutrophilia?
Neutrophilia is a medical condition characterized by high neutrophil count in the blood. It can be caused by various factors like infections, inflammation, and autoimmune disorders.
What is a left shift in neutrophils?
A left shift in neutrophils refers to an increase in the number of immature neutrophils in the blood, which is an indication of an ongoing infection or inflammation.
What is a differential white blood cell count?
A differential white blood cell count is a laboratory test that measures the percentage of different types of white blood cells, including neutrophils, in the blood.
How is a neutrophil count measured?
A neutrophil count is measured through a simple blood test. The blood sample is sent to a laboratory, where the number of neutrophils is counted and expressed as absolute neutrophil count (ANC) and percentage of the white blood cell (WBC) count.
How can I increase my neutrophil count?
A healthy diet, regular exercise, and adequate sleep can help maintain a healthy neutrophil count. If you have neutropenia, your healthcare provider may recommend medications or treatments to increase your neutrophil count.
How can I decrease my neutrophil count?
In general, decreasing the neutrophil count is not recommended as they are an essential part of the immune system. However, in certain medical conditions like autoimmune disorders, medications like corticosteroids can lower the neutrophil count.
References
- Peter J. Delves, Seamus J. Martin, Dennis R. Burton, and Ivan M. Roitt(2017). Roitt’s Essential Immunology, Thirteenth Edition. John Wiley & Sons, Ltd.
- Judith A. Owen, Jenni Punt, Sharon A. Stranford (2013). Kuby Immunology. Seventh Edition. H. Freeman and Company
- Klin Lab Diagn. 2006; (2):34-36.
- Rosales, C. (2018). Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Frontiers in Physiology, 9. doi:10.3389/fphys.2018.00113
- https://doi.org/10.1016/B978-1-4377-1738-9.00011-6
- Rosales C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol. 2018 Feb 20;9:113. doi: 10.3389/fphys.2018.00113. PMID: 29515456; PMCID: PMC5826082.
- https://www.healthline.com/health/neutrophils#anc
- https://www.nature.com/articles/3780067
- https://www.medicalnewstoday.com/articles/323982
- https://microbenotes.com/neutrophils/
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/neutrophil
- https://my.clevelandclinic.org/health/body/22313-neutrophils
- https://www.vedantu.com/biology/neutrophils