- Degradation of messenger RNA (mRNA) is an essential mechanism for controlling gene expression in bacterial cells.
- This process involves the coordinated action of a battery of cellular endonucleases and exonucleases, some of which are species-specific.
- They operate with the help of enzymes that covalently modify the 5′ or 3′ end of RNA or unwind base-paired regions.
- Initiated by events at either the 5′ terminus or an internal site, mRNA decay occurs at variable rates that are transcript-specific and governed by characteristics such as RNA sequence and structure, translating ribosomes, and bound sRNAs or proteins.
- Bacteria are able to orchestrate widespread changes in mRNA lifetimes in response to environmental cues by modulating the concentration or specific activity of cellular ribonucleases or by revealing the mRNA-degrading activity of cellular toxins.
mRNA Degradation in Prokaryotic Cells
- Critical to the survival of all living species is the ability to precisely regulate the expression of genetic information in order to make the proteins required to traverse the diverse environmental difficulties.
- This principle applies to both multicellular and bacterial organisms. Protein synthesis in bacterial cells can be regulated at any of three stages: transcription, translation, or mRNA degradation.
- The ability of cells to digest messenger RNA (mRNA) is an evolutionary necessity. The energetic costs of translation and the advantage of recycling ribonucleotides necessitate a system for swiftly eliminating useless transcripts.
- Importantly, high mRNA turnover confers a significant benefit by enabling cells to rapidly adapt protein synthesis to abrupt environmental stressors, whereas reaction times would be considerably longer if based exclusively on regulating transcription.
- Because mRNA degradation is not random, it contributes significantly to differential gene expression.
- The half-lives of specific mRNA transcripts in bacterial cells can range from seconds to approximately one hour, with proportional impacts on protein synthesis.
- The stability of translational units within the same polycistronic transcript can also vary, allowing co-transcribed genes to be produced at varying amounts. Changing mRNA degradation rates is frequently required for cells to respond to environmental signals.
Bacterial Ribonucleases
Bacteria use a vast arsenal of ribonucleolytic enzymes to degrade messenger RNA (mRNA), many of which are exclusive to certain bacterial clades.
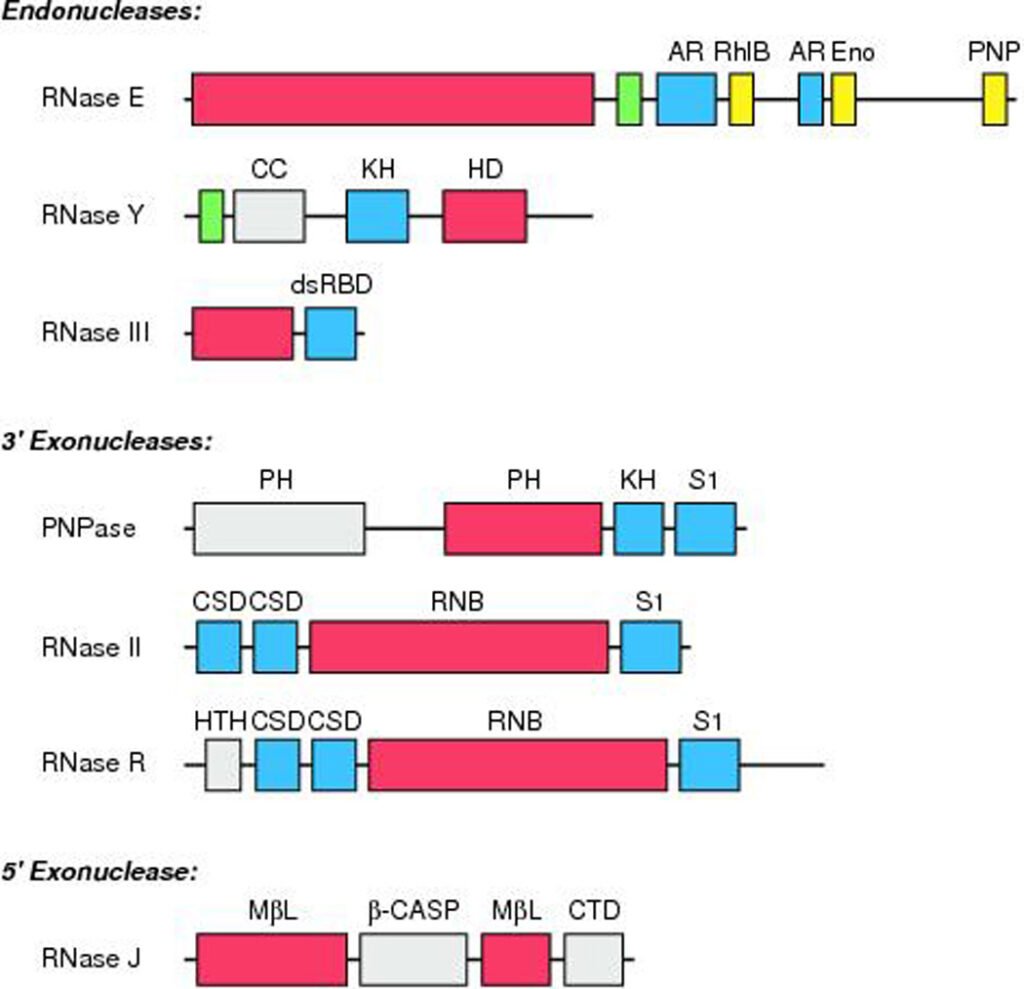
Endoribonucleases
RNase E, RNase Y, and RNase III are the most prominent endonucleases implicated thus far in bacterial mRNA turnover.
- RNase E and its homolog RNase G.: RNase E is one of the most significant bacterial ribonucleases for regulating mRNA degradation. This endonuclease was initially identified for its role in ribosomal RNA maturation in E. coli, but was later implicated in mRNA degradation when it was observed that the bulk mRNA stability and the half-lives of many individual transcripts increase significantly when a temperature-sensitive RNase E mutant is shifted to nonpermissive temperatures.
- RNase Y: In the absence of an RNase E homolog, RNase Y can function as the endonuclease that mediates mRNA degradation. This enzyme is composed of a transmembrane domain, an unstructured coiled-coil domain, an RNA-binding KH domain, and a catalytic HD domain. Although RNase Y and RNase E are structurally distinct, they share many properties. AU-rich single-stranded regions are cleaved internally and with low sequence specificity by both enzymes, which are membrane-associated and RNA-internally cleaved. In contrast to RNase E, the membrane-binding domain is necessary for the action of RNase Y.
- RNase III.: RNase III, as opposed to RNase E/G and RNase Y, cleaves RNA within double-stranded areas. RNase III plays a broad role in the maturation of ribosomal RNA and a selective involvement in the processing and destruction of messenger RNAs, small interfering RNAs, and CRISPR RNAs. Each subunit of RNase III is composed of an endonucleolytic domain and a double-stranded RNA-binding domain. The two centrally positioned catalytic sites function independently to cleave each strand of the RNA duplex, resulting in products with a distinctive 2-bp overhang at the 3 terminus. Although in vitro cleavage at a reduced rate has been seen for substrates as short as 11 bp (83, 129) biological substrates typically span at least two turns of an RNA helix or ∼20 b.
- Minor endonucleases: Other endoribonucleases whose primary function is tRNA synthesis have also been linked to the degradation of particular mRNAs. For instance, the ribonucleoprotein complex RNase P, which is essential for the maturation of tRNA 5 ends, targets noncoding sequences inside certain messages. RNase Z (RNase BN), which eliminates abnormal tRNA 3 ends in E. coli and appears to possess both endonuclease and 3 exonuclease activity, has also been linked to the degradation of a few mRNAs.
Exoribonucleases
In order to supplement the activity of cellular endonucleases, bacteria rely on a panel of exoribonucleases to destroy decay intermediates lacking protection at one or both termini. Generally, these exonucleases function processively with little or no sequence specificity.
- Phosphorolytic 3’ exonucleases: Either hydrolytically and irreversibly to generate nucleoside monophosphate products or phosphorolytically (using orthophosphate as a nucleophile) to generate nucleoside diphosphates in a reversible reaction is how bacterial 3′ exoribonucleases function. Currently, all known phosphorolytic 3′ exonucleases belong to the PDX enzyme family. This family’s prototypical members include polynucleotide phosphorylase (PNPase) and RNase PH. The former is substantially implicated in mRNA decay, whereas the latter has been researched mostly in relation to tRNA maturation and appears to have just a modest role in mRNA decay. PNPase possesses both degradative and synthetic capacities, consistent with the nature of the reversible phosphorolytic reaction it catalyses. It can degrade RNA from 3′ to 5′ and add a heteropolymeric tail to the 3′ end of RNA in vitro. Both of these processes contribute to mRNA degradation in vivo. As an exonuclease, PNPase degrades RNAs with a single-stranded 3′ end preferentially. As a polymerase, PNPase can add adenine-rich single-stranded tails that facilitate the 3-exonucleolytic destruction of organised RNA sections.
- Hydrolytic 3’ exonucleases: The predominant hydrolytic 3 exoribonucleases in bacterial cells are RNR superfamily members. As irreversible reaction catalysts, they serve entirely as degradative enzymes. E. coli, like the majority of other -proteobacteria, possesses two exonucleases, RNase II and RNase R. It can survive the lack of either of these enzymes or PNPase alone, but coupled mutations that delete PNPase in conjunction with RNase II or RNase R are synthetically fatal.
- 5’ exonucleases: The revelation that RNase J can progressively remove nucleotides from the 5′ end of RNA, with a strong preference for 5′ monophosphorylated substrates, contradicted the long-held notion that 5′ exoribonucleases do not exist in bacteria. This enzyme is a dimer of dimers with each subunit containing a bipartite metallo—lactamase domain, a -CASP domain, and a carboxy-terminal domain. It is absent in E. coli and was initially found in B. subtilis as an endonuclease. At each dimer interface, an RNA-binding channel extends deep inside the protein to a catalytic active site where a monophosphorylated 5′ end, but not a triphosphorylated 5′ end, can bind in order to position the 5-terminal nucleotide for hydrolytic elimination. The channel continues past the catalytic centre and emerges on the other side of the enzyme, which explains RNase J’s ability to function as both a 5′ exonuclease and an endonuclease.
Oligoribonucleases
Oligoribonuclease, a hydrolytic 3′ exoribonuclease, differs from other bacterial exonucleases in a fundamental way.
- This enzyme has a strong predilection for RNA substrates that are no longer than five nucleotides.
- It is essential for RNA breakdown.
- Because their architectures and processes prohibit them from entirely degrading their substrates, PNPase, RNase II, and RNase R generate 2- to 5-nucleotide-long 5-terminal oligonucleotides as reaction products.
- Oligoribonuclease transforms these oligonucleotides into mononucleotides, thereby replenishing the cellular pool of RNA precursors and preventing their inclusion at the 5 end of new transcripts.
- Oligoribonuclease is vital in E. coli, where it is the only ribonuclease capable of degrading oligonucleotides efficiently; however, a sequence homolog of the E. coli enzyme (Orn) is not present in all bacterial species.
- It has been demonstrated that some organisms lacking this enzyme contain a different ribonuclease (NrnA/B or NrnC) with similar characteristics.
- Other species may include ribonucleases that fulfil this activity but have not yet been identified.
RNA Degradosomes
- Enzymes essential for mRNA decay frequently assemble into a multimeric complex called an RNA degradosome, presumably to improve their degradative effectiveness.
- Typically, these degradosomes contain at least one ribonuclease and an RNA helicase.
mRNA Degradation Pathways in Bacterial Cells
- Despite the diversity of ribonucleases seen in bacteria, the fundamental processes of mRNA degradation are strikingly similar amongst species.
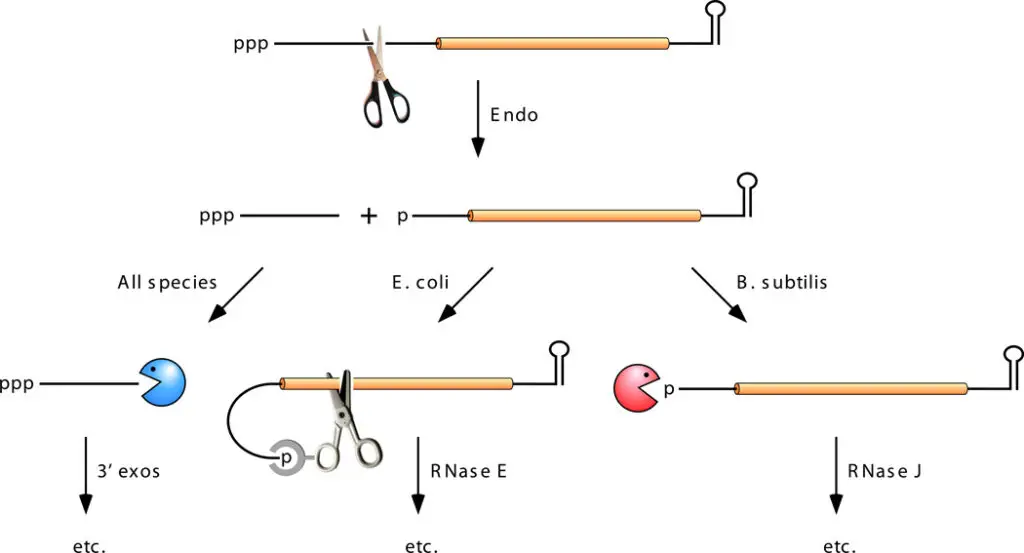
- There appear to be two initiation pathways for mRNA degradation.
- In one (direct access), degradation begins with ribonuclease attack, while in the other (5-end-dependent access), the 5′-terminal triphosphate is converted to monophosphate first.
Direct-Access Pathway
- Internal cleavage by an endonuclease is the first degradative process in the direct-access pathway.
- In E. coli and comparable organisms, this phase is often catalysed by RNase E; however, it has been demonstrated that for certain mRNAs, other endonucleases initiate decay.
- In contrast, in species lacking RNase E, such as B. subtilis, internal cleavage by RNase Y generally precedes destruction.
- This initial cleavage generates 5′- and 3′-terminal mRNA fragments, each of which normally has a lower half-life than the full-length transcript.
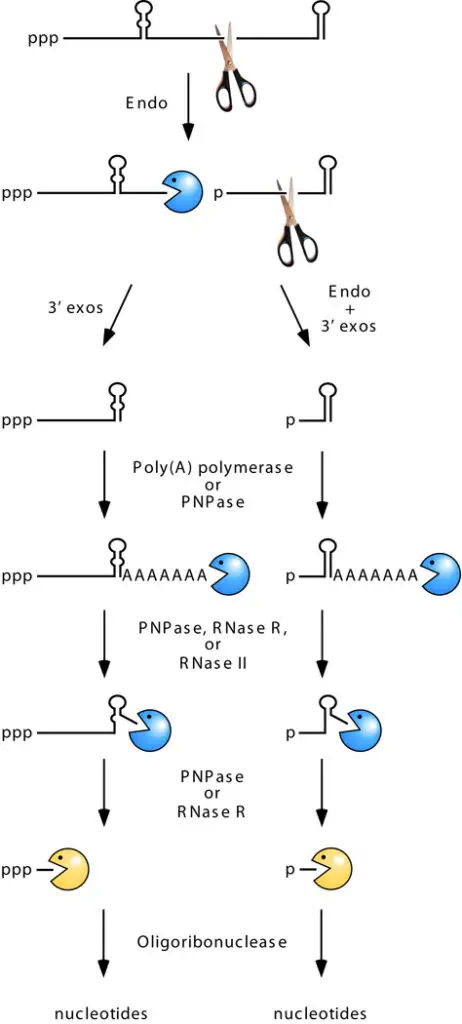
- In the majority of instances, the 5 fragment generated by endonucleolytic cleavage lacks a protective stem-loop at its 3 end, making it prone to rapid 3′-exonucleolytic disintegration. Despite the multiple hurdles that 3′ exonucleases may face, this degradation frequently progresses to completion.
- Despite the fact that thermodynamically robust base pairing normally impedes exonucleolytic breakdown, such barriers can be overcome with the assistance of an enzyme that appends a single-stranded tail downstream of the hindrance.
- Poly(A) polymerase (PAP), which can polyadenylate the 3 end of decay intermediates from which a 3 exonuclease has disengaged, is principally responsible for tailing in E. coli.
- A-rich tails can be inserted by the template-independent polymerase activity of a phosphorolytic exonuclease such as PNPase in bacterial species lacking a poly(A) polymerase.
- Successive rounds of poly(A) addition and removal downstream of a base-paired structure provide repeated opportunities for barrier penetration by PNPase (with the assistance of RhlB) or RNase R, allowing exonucleolytic degradation to proceed past the structured area.
- Due to its stringent specificity for single-stranded 3′ ends, RNase II can delay the exonucleolytic destruction of stem-loop structures by unproductively eliminating the poly(A) tail on which PNPase and RNase R depend without ever harming the stem-loop itself (64).
- Therefore, 3′ -exonucleolytic penetration of such structures is typically slower than upstream endonucleolytic cleavage, particularly when they are thermodynamically stable and positioned in an untranslated region.
- In the process of degrading 5′-terminal mRNA fragments, 3′ exonucleases may encounter ribosomes that are translating in the opposite direction.
- To rescue ribosomes halted at the 3′ end of degradation intermediates without a termination codon, a specialised bacterial RNA (tmRNA) with characteristics of both tRNA and mRNA and its protein escort are recruited (SmpB).
- SmpB enables the transition of the ribosome template from the shortened mRNA to the tmRNA, which carries a termination codon that allows the ribosome to be released.
- RNase R then destroys the mRNA fragment beginning at its newly exposed 3′ end.
- Despite having a stem loop that shields it from 3′ -exonucleolytic degradation, the 3′ fragment formed by the initial endonucleolytic cleavage is often extremely unstable due to its monophosphorylated 5′ terminus.
- In bacterial species containing RNase J, the presence of only one phosphate at that end makes such intermediates susceptible to rapid 5′-exonucleolytic destruction.
- In the absence of RNase J, these decay intermediates are swiftly degraded by RNase E, whose ribonucleolytic activity is significantly increased when the 5′ end of a substrate is monophosphorylated.
- Repeated cleavage by this endonuclease generates mRNA fragments susceptible to exonucleolytic degradation from an unprotected 3′ end or, in the case of the 3′-terminal fragment bearing the terminator stem-loop of the original transcript, to degradation by a mechanism involving polyadenylation and 3 -exonucleolytic attack.
3’ -Exonucleolytic Initiation of Decay
- Inactivating RNase E in E. coli delays but does not eliminate mRNA decay, revealing the existence of other, RNase E–independent degradation processes.
- Several transcripts whose degradation is inhibited by RNase E inactivation are further maintained when cells additionally lack PAP or PNPase.
- These findings show that poly(A)-dependent 3′ -exonucleolytic degradation can begin mRNA decay on occasion.
- However, the fact that the influence of PAP and PNPase is typically minimal in the presence of RNase E suggests that 3′-exonucleolytic beginning of decay is typically considerably slower than other pathways.
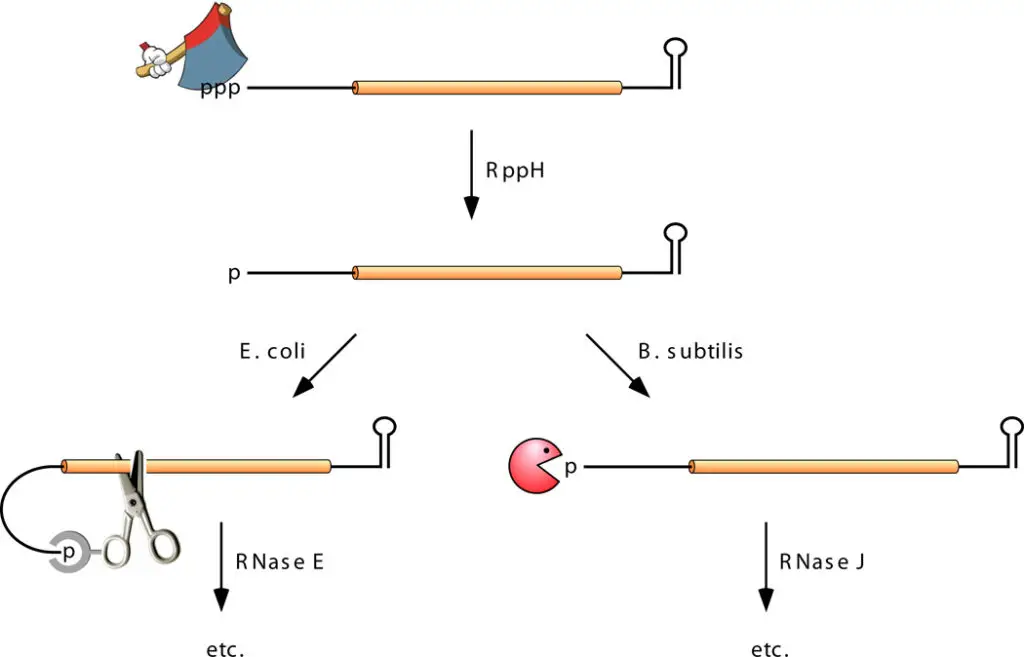
5’-End-Dependent Pathway
- The direct-access mechanism for endonucleolytic initiation does not explain the ability of a 5′-terminal stem-loop to stabilise numerous transcripts, despite its relevance to the degradation of a substantial proportion of primary transcripts.
- This observation led to the discovery and characterization of a separate, 5′-end-dependent mRNA degradation pathway in which endonucleolytic cleavage is not the initial event. Rather, this pathway is activated by a non-nucleolytic process that marks transcripts for high turnover: the conversion of the 5 terminal from triphosphate to monophosphate.
- This alteration, catalysed by the RNA pyrophosphohydrolase RppH, dramatically enhances the vulnerability of mRNA to breakdown by RNase E or RNase J, which both attack monophosphorylated RNA substrates aggressively.
- When the rppH gene is deleted in E. coli, the steady-state concentration of hundreds of mRNAs increases considerably, showing that a substantial amount of the transcriptome is degraded by the 5′-end-dependent pathway.
- As RppH, RNase E, and RNase J can only interact with single-stranded 5′ ends, the identification of the mechanism of 5′-end-dependent degradation explains the protective effect of 5′-terminal stem-loops.
- Indeed, biochemical analyses of RppH from B. subtilis and E. coli reveal that enzyme requires a minimum of two and ideally three or more unpaired nucleotides at the 5′ end of its substrates.
- In addition, B. subtilis RppH has a tight requirement for guanylate as the second nucleotide, but E. coli RppH does not.
- However, 5′- end-dependent mRNA degradation in B. subtilis does not depend solely on the identity of the second nucleotide or even on RppH, presumably due to the existence of an undiscovered RNA pyrophosphohydrolase in that organism.
- In contrast, there is no indication that E. coli have an alternate pyrophosphate-removing enzyme.
References
- Hui MP, Foley PL, Belasco JG. Messenger RNA degradation in bacterial cells. Annu Rev Genet. 2014;48:537-59. doi: 10.1146/annurev-genet-120213-092340. Epub 2014 Oct 1. PMID: 25292357; PMCID: PMC4431577.
- Penalva, L.O.F. (2013). mRNA Degradation. In: Dubitzky, W., Wolkenhauer, O., Cho, KH., Yokota, H. (eds) Encyclopedia of Systems Biology. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-9863-7_318
- Beelman, C. A., & Parker, R. (1995). Degradation of mRNA in eukaryotes. Cell, 81(2), 179–183. doi:10.1016/0092-8674(95)90326-7
- Rauhut, R., & Klug, G. (1999). mRNA degradation in bacteria. FEMS Microbiology Reviews, 23(3), 353–370. doi:10.1111/j.1574-6976.1999.tb00404.x
- Deneke, Carlus. (2012). Theory of mRNA degradation.
- Hui, M. P., Foley, P. L., & Belasco, J. G. (2014). Messenger RNA Degradation in Bacterial Cells. Annual Review of Genetics, 48(1), 537–559. doi:10.1146/annurev-genet-120213-092340
- https://reactome.org/content/detail/R-HSA-429958
- https://sciencing.com/degradation-mrna-6196816.html
- http://www.eb.tuebingen.mpg.de/remco-sprangers/mrna-degradation.html
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.