What is Most Probable Number (MPN) Test?
- The Most Probable Number (MPN) test is a statistical method used to estimate the concentration of viable microorganisms in a sample. It is based on the random dispersion of microorganisms per volume in a given sample. This method involves adding measured volumes of water to a series of tubes containing a liquid indicator growth medium. Tubes that receive one or more indicator bacteria will show growth and a characteristic color change, while those without indicator bacteria will not. The number and distribution of positive and negative reactions are then used to estimate the MPN of indicator organisms in the sample by referencing statistical tables.
- The MPN test is carried out in three steps: the presumptive test, the confirmed test, and the completed test. This sequential approach helps ensure accuracy and reliability in estimating microbial populations.
- MPN is commonly used to estimate microbial populations in soils, waters, and agricultural products. It is particularly useful for samples containing particulate material that interferes with plate count methods. In water quality testing, MPN is employed to determine the presence of fecal coliforms, which indicate fecal contamination. The presence of few fecal coliform bacteria suggests that the water is likely free of disease-causing organisms, whereas a large number of these bacteria indicates a high probability of harmful pathogens, making the water unsafe for consumption.
- The coliform group includes all aerobic and facultative anaerobic, gram-negative, non-spore-forming, rod-shaped bacteria that ferment lactose with gas and acid formation within 48 hours at 37°C. The standard test for coliforms can be performed using the multiple tube fermentation technique or the membrane filter technique. In the multiple-tube fermentation method, bacteria’s ability to ferment nutrient broth (like lactose) to produce gas and acid within 48 hours at 37°C is used to detect coliform bacteria. Fecal coliform presence is differentiated by their ability to produce gas in a shorter period (24 hours) and at a higher temperature (44°C).
- This method requires testing three sets of dilutions, each set containing five samples of the same strength and volume. Results are reported in terms of the Most Probable Number (MPN) of organisms present, which is an estimate of the mean density of coliforms in the sample. An MPN of 10, for instance, means 10 coliforms per 100 ml of water. The precision of the test depends on the number of tubes used. Optimal results are obtained when the largest sample inoculum shows gas in some or all tubes, and the smallest sample inoculum shows no gas in all or most tubes. Bacterial density is then estimated using tables based on the number of positive tubes in the multiple dilutions.
Definition of Most Probable Number (MPN) Test
The Most Probable Number (MPN) test is a statistical method used to estimate the concentration of microorganisms, particularly indicator organisms, in a sample by analyzing positive and negative reactions in a series of tests. It is commonly employed in water quality analysis to assess contamination levels.
Principle of Most Probable Number Test (MPN Test)
The Most Probable Number (MPN) test is a vital method in microbiology used to estimate the concentration of viable microorganisms in a water sample. This test relies on serial dilution and inoculation of the sample into lactose broth. If coliform bacteria are present, they will utilize the lactose, producing acid and gas as metabolic byproducts. The acid causes a color change in the medium, while gas production is evident from bubbles collected in an inverted Durham tube within the medium.
The principle of the MPN test involves three key steps:
- Presumptive Test: In this initial step, multiple tubes containing lactose broth are inoculated with serial dilutions of the water sample. Tubes that show a color change and gas production are considered positive for coliforms.
- Confirmatory Test: Positive tubes from the presumptive test are further tested to confirm the presence of coliform bacteria. This is done by transferring a sample from the positive tubes to a new set of media that is more selective for coliforms. The presence of acid and gas in these tubes confirms the presence of coliform bacteria.
- Completed Test: The final step involves isolating and identifying the specific coliform bacteria. Samples from the confirmatory test are streaked onto selective agar plates. Colonies that grow on these plates are then tested further to confirm they are coliforms.
The MPN is determined by counting the number of tubes that show a positive reaction (both color change and gas production) and comparing the pattern of positive results across different dilutions to standard statistical tables. These tables provide an estimate of the number of coliforms in the original sample.
This method is particularly useful for assessing water quality. It helps determine if water is contaminated with fecal coliforms, which are indicators of potential pathogenic organisms. The presence of coliform bacteria, especially in large numbers, suggests that the water may contain harmful pathogens, making it unsafe for consumption.
The MPN test’s detailed and sequential approach ensures accuracy in detecting and estimating microbial populations in water samples. Its reliance on specific metabolic properties of coliform bacteria allows for precise identification and quantification, making it an essential tool in environmental microbiology and public health.
Objectives of Most Probable Number Test (MPN Test)
- Enumeration of Bacteria in Drinking Water: The foremost objective of the MPN test is to enumerate, or count, the number of bacteria present in a drinking water sample. This is achieved through the MPN method, which involves serial dilution of the water sample and its subsequent inoculation in a specific growth medium. By observing the reactions in this medium, such as color changes and gas production, scientists can estimate the concentration of viable bacteria in the original sample. Therefore, this method provides a quantitative analysis of bacterial presence, ensuring that drinking water meets the safety standards set for microbial content.
- Identification of Bacteria in the Drinking Water Sample: Besides quantifying the bacterial content, the MPN test also aims to identify the specific types of bacteria present in the drinking water sample. This is crucial because not all bacteria are harmful; some are benign or even beneficial. However, certain bacterial strains can pose health risks if consumed. By identifying the bacteria, one can ascertain whether the water is contaminated with potentially harmful microbial species. This identification is typically achieved through further tests and analyses, which might involve studying the morphology, biochemical characteristics, and genetic makeup of the isolated bacteria.
MPN Test Procedure
MPN test is performed in 3 steps
- Presumptive test
- Confirmatory test
- Completed test
1. Presumptive Test of MPN Test
- The presumptive test is the initial step in the Most Probable Number (MPN) test, specifically designed to detect coliform bacteria in water samples. This enrichment procedure is conducted using fermentation tubes filled with a selective growth medium, commonly MacConkey lactose broth. These tubes contain inverted Durham tubes to capture gas produced during fermentation.
- The process begins by inoculating a series of lactose broth tubes with measured amounts of the water sample. Typically, this series comprises multiple groups, each containing three, five, or more tubes. The main components of the medium include lactose, which serves as a fermentable sugar, a surfactant like Na-lauryl sulfate or Na-taurocholate (bile salt), and a pH indicator dye, such as bromcresol purple or brilliant green.
- Lactose is a selective factor because many bacteria cannot ferment it, whereas coliform bacteria and a few other bacterial types can. The surfactant and dye do not inhibit coliform bacteria but are effective against other bacteria, including spore formers. Therefore, the presence of coliform bacteria is indicated by acid and gas production, leading to a color change in the medium and gas accumulation in the Durham tube.
- The presumptive test aims to identify potential coliform contamination by observing these reactions. Positive results, indicated by both a color change and gas production, suggest the presence of coliform bacteria. These positive tubes are then used in subsequent confirmatory tests to verify the presence of coliforms.
Requirements for Presumptive Test
Medium
- Lactose Broth: A nutrient-rich medium that supports the growth of coliform bacteria, allowing them to ferment lactose and produce gas.
- MacConkey Broth: Contains bile salts and crystal violet to inhibit non-coliform bacteria, alongside lactose for fermentation.
- Lauryl Tryptose (Lactose) Broth: A selective medium that promotes coliform growth while inhibiting other bacteria.
Glassware
- Test Tubes:
- 20 ml Capacity: Used for larger volume samples to increase sensitivity.
- 10 ml Capacity: Standard size for medium volume samples.
- 5 ml Capacity: Used for smaller volume samples or higher concentrations of bacteria.
- Durham Tubes: Small inverted tubes placed inside the test tubes to capture gas produced during lactose fermentation.
Others
- Sterile Pipettes: Essential for accurately measuring and transferring sample volumes into the test tubes to avoid contamination.
Additional Details
- Selective Agents:
- Surfactants: Such as sodium lauryl sulfate, used in some media to inhibit non-coliform bacteria.
- pH Indicator Dyes: Like bromcresol purple or brilliant green, which change color in response to acid production from lactose fermentation.
- Incubation Conditions:
- Temperature: Typically 35°C to promote optimal growth of coliform bacteria.
- Duration: Generally 24-48 hours to allow sufficient time for fermentation and gas production.
Media Preparation
The preparation of the medium for the Most Probable Number (MPN) test involves several key steps to ensure accurate detection of coliform bacteria. The media used can be either MacConkey broth or lactose broth, prepared in specific concentrations. The following steps outline the procedure:
Media Preparation
- Concentration Preparation:
- Single Strength Medium: This is the standard concentration used for testing. Prepare it according to the manufacturer’s instructions.
- Double Strength Medium: This concentration is more concentrated than the single strength. Prepare it by doubling the amount of each component specified for the single strength medium.
- For Untreated or Polluted Water:
- Double Strength Medium:
- Dispense 10 mL of double strength medium into each of 10 test tubes.
- Single Strength Medium:
- Dispense 10 mL of single strength medium into each of 5 test tubes.
- Durham Tubes:
- Add a Durham tube to each test tube in an inverted position. This allows for the detection of gas production.
- Double Strength Medium:
- For Treated Water:
- Double Strength Medium:
- Dispense 10 mL of double strength medium into each of 5 test tubes.
- Single Strength Medium:
- Dispense 50 mL of single strength medium into a single bottle.
- Durham Tubes:
- Add a Durham tube in an inverted position to each test tube and the bottle.
- Double Strength Medium:
Final Preparation Steps
- Inspection:
- Ensure that the Durham tubes are correctly positioned and that there are no air bubbles in the inner vial of the Durham tubes.
- The inner vial should be completely filled with liquid.
- Sterilization:
- Sterilize the prepared medium by autoclaving.
- Set the autoclave to 15 lbs pressure (121°C) and sterilize for 15 minutes.
Procedure of Presumptive Test
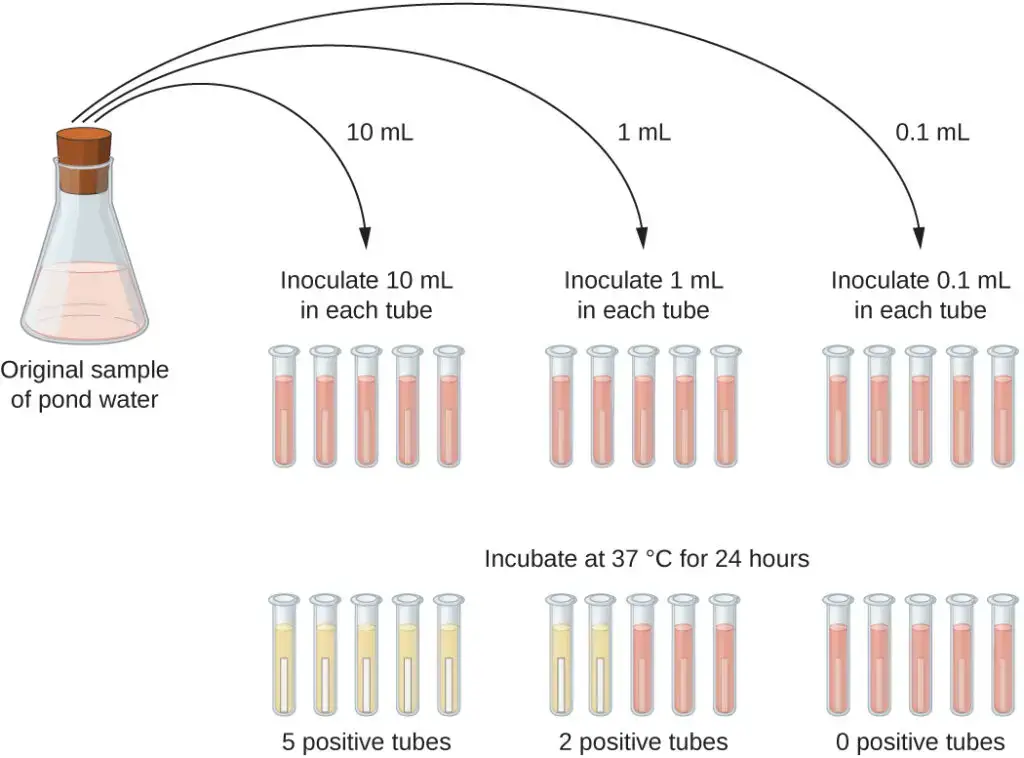
The presumptive test is the initial step in the MPN test, aimed at estimating the number of coliform bacteria in water samples. This test involves inoculating a series of tubes with the water sample and examining the results for signs of coliform presence. The procedure differs slightly for untreated (polluted) and treated (unpolluted) water.
For Untreated (Polluted) Water
- Setup:
- Prepare 5 tubes with double strength medium and 10 tubes with single strength medium for each water sample.
- Inoculation:
- Add 10 mL of the water sample to each of the 5 tubes containing double strength medium using a sterile pipette.
- Add 1 mL of the water sample to each of 5 tubes containing single strength medium.
- Add 0.1 mL of the water sample to each of the remaining 5 tubes containing single strength medium.
- Incubation:
- Incubate all tubes at 37°C for 24 hours.
- If no positive results are observed, re-incubate the tubes for an additional 24 hours (up to 48 hours in total).
- Results and Interpretation:
- Evaluate the tubes for positive reactions, indicated by color changes and gas production in the Durham tubes.
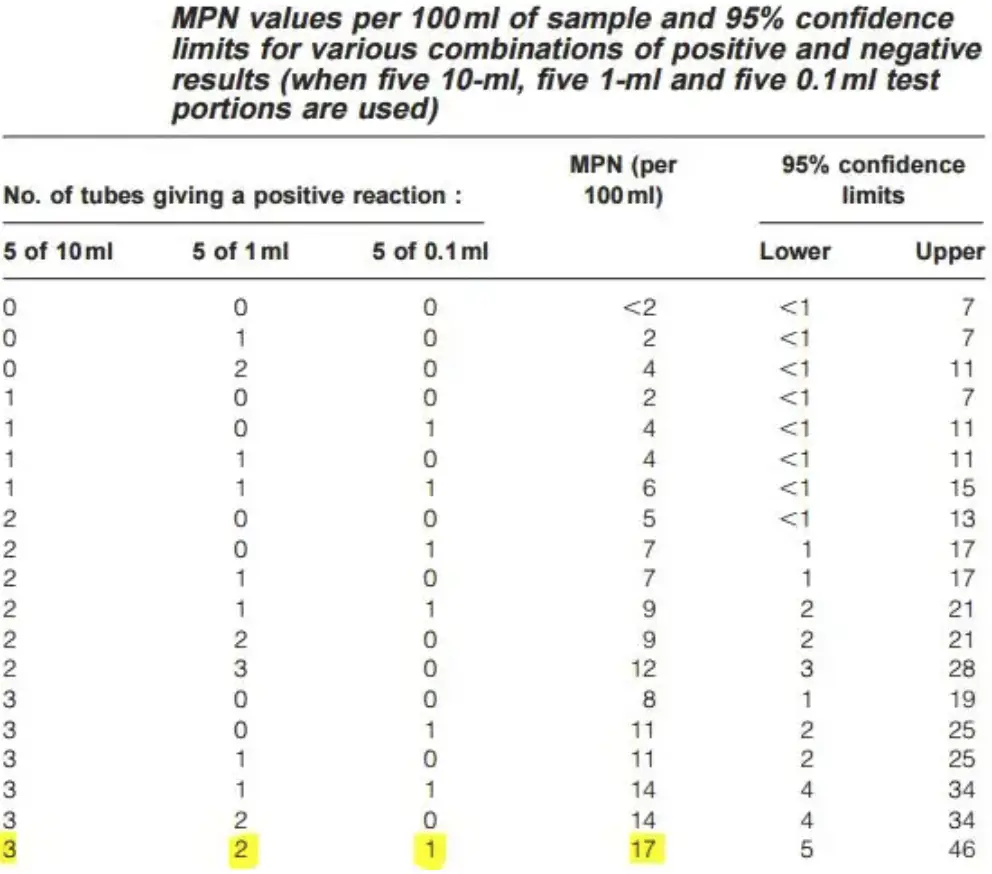
- Compare the pattern of positive results to a standard statistical chart to estimate the number of coliform bacteria. For example, a pattern of 3–2–1 (3 × 10 mL positive, 2 × 1 mL positive, 1 × 0.1 mL positive) corresponds to an MPN value of 17, suggesting an estimated 17 coliforms per 100 mL of water.
For Treated (Unpolluted) Water
- Setup:
- Prepare 1 tube with 50 mL of single strength medium and 5 tubes with 10 mL of double strength medium for each water sample.
- Inoculation:
- Add 50 mL of the water sample to the single strength medium tube.
- Add 10 mL of the water sample to each of the 5 tubes containing double strength medium.
- Incubation:
- Incubate all tubes at 37°C for 24 hours.
- If no positive reactions are observed, re-incubate for an additional 24 hours (up to 48 hours in total).
- Results and Interpretation:
- Observe the tubes for positive reactions. Record the number of positive tubes.
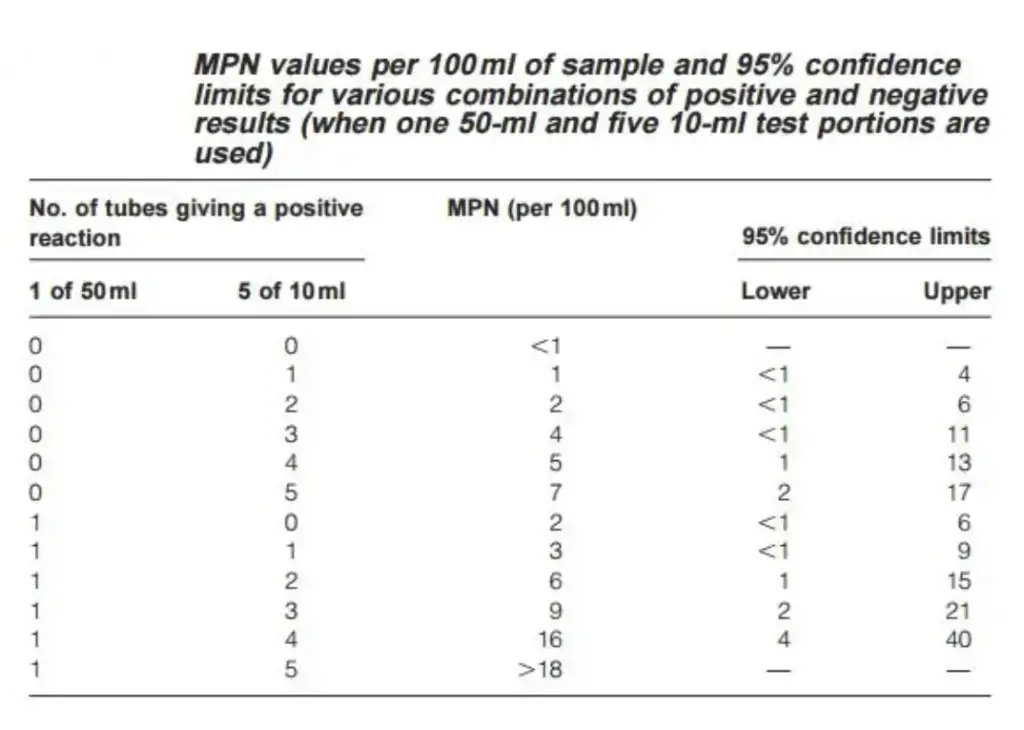
- Compare the pattern of positive results to a standard chart to determine the MPN value. For instance, a result of 1-4 (1 × 50 mL positive, 4 × 10 mL positive) yields an MPN value of 16, indicating an estimated 16 coliforms per 100 mL of water.
Presumptive Test Result
Positive Result
- Gas Production:
- A positive result is indicated by the formation of 10% or more gas in the Durham tube. This gas production must occur within 24 to 48 hours of incubation.
- Turbidity:
- Turbidity in the growth medium also signifies a positive result. This cloudiness indicates bacterial growth.
- Color Change:
- The medium may change color, further confirming a positive result. This color shift is due to the fermentation of lactose by coliform bacteria, producing acid and altering the pH.
- Interpretation:
- The combination of gas production, turbidity, and color change suggests the presence of coliform bacteria, which may indicate fecal contamination.
Negative Result
- Absence of Gas:
- No gas formation in the Durham tube points to a negative result. This lack of gas implies that coliform bacteria are not present.
- No Turbidity:
- If there is no turbidity in the growth medium, it suggests that bacterial growth did not occur.
- No Color Change:
- Absence of color change in the medium indicates no lactose fermentation and thus, no coliform bacteria present.
- Interpretation:
- A negative result, characterized by no growth, gas formation, or color change, implies the absence of coliform bacteria.
Considerations
- Presumptive Nature:
- It is important to note that the test is presumptive. Other bacteria can produce similar results, such as gas formation and turbidity, without being coliforms. Therefore, further confirmatory testing is required to definitively identify coliform bacteria.
| Result | Description |
|---|---|
| Positive | – Formation of 10% gas or more in the Durham tube within 24 to 48 hours. – Turbidity in the growth medium. – Color change in the medium. – Indicates the presence of coliform bacteria and suggests the possibility of fecal pollution. |
| Negative | – No visible growth observed in the growth medium. – No gas formation in the Durham tube. – Indicates the absence of coliform bacteria.<br>- Suggests a lower likelihood of fecal pollution. – Note: Does not guarantee absence of other microorganisms. |
2. Confirmatory Test of MPN Test
- The confirmatory test in the Most Probable Number (MPN) test is conducted to verify the presence of coliform bacteria when a presumptive test yields positive or doubtful results. This step ensures the accuracy and reliability of the initial findings.
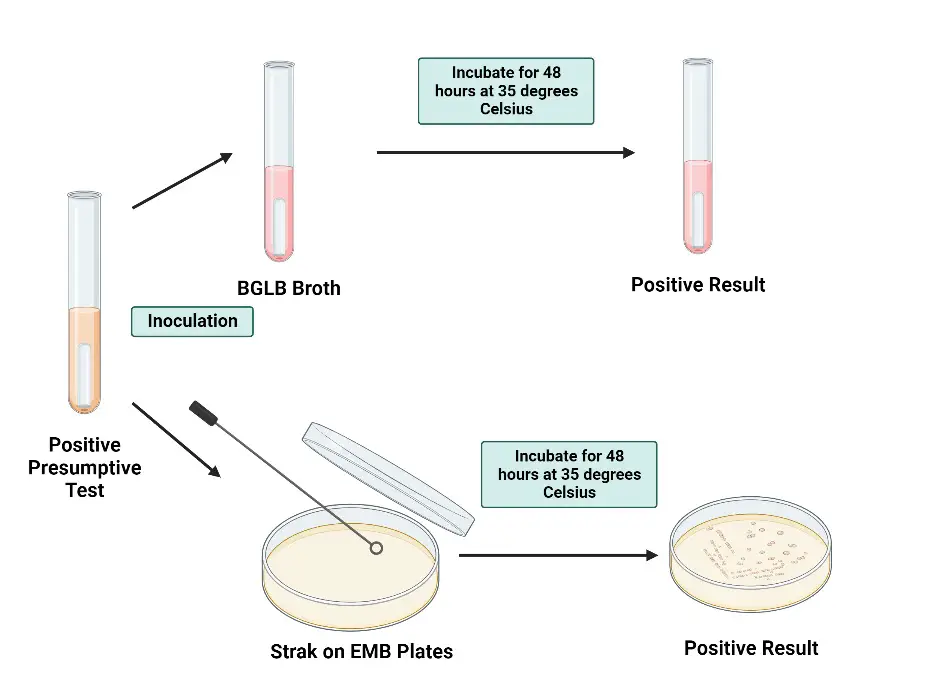
- First, a loopful of growth from a positive presumptive tube is transferred to a tube of brilliant green lactose bile (BGLB) broth, a selective medium for coliform detection in water, dairy, and other food products. The broth, containing lactose and a selective agent, is incubated at 35°C for 48 hours. A Durham tube within the broth detects gas production, which indicates the fermentation of lactose by coliform bacteria.
- Simultaneously, a loopful of growth from a positive presumptive tube is streaked onto a plate of LES Endo agar or EMB agar. This plate is incubated at 35°C for 18–24 hours. On this medium, typical coliform bacteria such as Escherichia coli and Enterobacter aerogenes exhibit good growth, forming red to black colonies with dark centers or a metallic sheen. In contrast, Salmonella typhi grows well but forms colorless colonies, while Staphylococcus aureus growth is inhibited.
- This confirmatory test process is essential for distinguishing true coliform bacteria from other microorganisms that might have given a false positive in the presumptive test. By using selective media and observing specific growth patterns and gas production, the test provides a robust verification method.
Procedure of Confirmatory Test
Testing of positive presumptive in BGLB medium
The Brilliant Green Lactose Bile Broth (BGLB) medium is a specialized medium used to confirm the presence of coliform bacteria in water samples. The procedure for testing positive presumptive samples in BGLB medium is detailed and systematic, ensuring accurate results.
- Preparation of BGLB Medium:
- Begin by preparing a solution of BGLB medium. The components required are as follows:
- Peptone: 10 g
- Lactose: 10 g
- Bile salt: 20 g
- Brilliant green: 0.0133 g
- Distilled water: 1 L
- Once the components are combined, the next step is to sterilize the medium. This is achieved by autoclaving the BGLB medium for 15 minutes at a temperature of 121 degrees Celsius.
- Begin by preparing a solution of BGLB medium. The components required are as follows:
- Inoculation of the Medium:
- Before inoculation, it’s essential to shake the positive presumptive tubes gently to ensure an even distribution of the bacteria.
- Using a sterile loop, transfer a loopful of culture from the positive presumptive tube into the BGLB fermentation tube. It’s crucial to note that the brilliant green dye present in the BGLB medium serves a specific function. This dye inhibits the growth of gram-positive bacteria, ensuring that only the desired coliform bacteria grow in the medium.
- Incubation:
- After inoculation, the test tubes are incubated at a consistent temperature of 35 degrees Celsius. This incubation period lasts for 48 hours, allowing sufficient time for the bacteria to grow and produce observable results.
- Observation:
- Post the incubation period, the test tubes containing the BGLB medium and the inoculum from the positive presumptive test are closely observed. The primary indicator of coliform presence is the production of gas in the inverted Durham tube. This gas production is a result of the fermentation process of the coliform bacteria.
- Result Interpretation:
- The results are interpreted based on the observations made. If gas production is observed in the BGLB medium, it indicates the presence of coliforms in the sample. This gas production is a definitive sign of coliform bacteria, as they are known to ferment lactose, producing gas in the process.
Therefore, the BGLB medium serves as an effective tool in the confirmation of coliform bacteria in water samples. The systematic procedure ensures that the results are both accurate and reliable, providing a clear indication of water quality.
Testing of positive presumptive in EMB medium
Eosin Methylene Blue (EMB) agar medium is a selective and differential medium primarily used to isolate and differentiate coliforms from other bacterial species. The procedure for testing positive presumptive samples in EMB medium is meticulously structured to ensure the accurate identification of coliform bacteria.
- Preparation of EMB Medium:
- Begin by formulating the EMB agar medium using the following components:
- Peptone: 10 g
- Agar: 15 g
- Lactose: 10 g
- Eosin Y: 0.4 g
- Methylene blue: 0.065 g
- Dipotassium hydrogen phosphate: 2 g
- Distilled water: 1L
- After combining these components, the next step is sterilization. Sterilize the EMB agar by autoclaving it for 15 minutes at 121 degrees Celsius.
- Begin by formulating the EMB agar medium using the following components:
- Pouring the Agar:
- Once sterilized, pour the molten EMB agar onto sterilized Petri plates. Allow the medium to solidify, creating a firm surface suitable for bacterial growth.
- Inoculation of the Medium:
- Prior to inoculation, shake the positive presumptive tubes gently to ensure a uniform distribution of bacteria.
- Using a sterile loop, streak a loopful of culture from the positive presumptive tube onto the solidified EMB agar surface.
- Incubation:
- Place the inoculated Petri plates in an incubator set at 35 degrees Celsius. Allow the plates to incubate for 48 hours, providing adequate time for bacterial growth and differentiation.
- Observation:
- After the incubation period, examine the Petri plates for bacterial colonies on the EMB medium. Typically, three distinct types of colonies can develop:
- Typical colony: These colonies are characterized by their nucleated appearance and may exhibit a metallic sheen. This sheen is indicative of coliform bacteria, which ferment lactose, producing acid that reacts with the dyes in the medium.
- Atypical colonies: These colonies appear non-nucleated, opaque, and mucoid. They represent bacterial species that do not produce the characteristic sheen.
- Negative colonies: These colonies differ in appearance from both typical and atypical colonies and represent other bacterial species.
- After the incubation period, examine the Petri plates for bacterial colonies on the EMB medium. Typically, three distinct types of colonies can develop:
- Result Interpretation:
- The presence of typical colonies with a metallic sheen on the EMB agar is a clear indication of coliform bacteria. This sheen is a result of the interaction between the acid produced by lactose fermentation and the dyes in the EMB medium.
Therefore, the EMB agar medium serves as a crucial tool in the microbiological analysis of water samples. Its selective and differential properties ensure the accurate identification and differentiation of coliform bacteria from other microbial species.
Confirmed Test Result
- Positive Result:
- Gas Formation in Lactose Broth: One of the primary indicators of a positive result is the formation of gas in the lactose broth. This gas formation signifies the fermentation of lactose by bacteria, which is a characteristic trait of coliforms.
- Observation on EMB Agar: On Eosin Methylene Blue (EMB) agar, coliform bacteria produce distinctive colonies that exhibit a greenish metallic sheen. This sheen is a hallmark of coliform bacteria and differentiates them from non-coliform bacteria, which do not produce such a sheen.
- Presence of Thermotolerant E.coli: The growth of colonies at elevated temperatures, specifically around 44.5 ±0.2°C, indicates the presence of thermotolerant E.coli, a subset of coliforms. These bacteria can survive and thrive at higher temperatures, making their detection crucial for water safety assessments.
- Negative Result:
- Absence of Gas Formation: If there is no gas formation observed in the lactose broth, it suggests that the sample does not contain coliform bacteria or they are present in negligible amounts.
- Observation on EMB Agar: A negative result is also indicated by the absence of coliform-like colonies on the EMB agar. Specifically, if there are no colonies exhibiting the characteristic greenish metallic sheen, it suggests that coliforms are not present in the sample.
| Result | Description |
|---|---|
| Positive | – Formation of gas in lactose broth medium. – Presence of colonies with a greenish metallic sheen on Eosin Methylene Blue (EMB) agar medium. – Indicates the presence of coliform bacteria or a member of the coliform group in the water sample. – High-temperature presence of typical colonies suggests the presence of thermotolerant Escherichia coli (E.coli). |
| Negative | – Absence of gas formation in lactose broth medium. – Failure to demonstrate coliform-like colonies (no greenish metallic sheen) on EMB agar medium. – Indicates the absence of coliform bacteria in the water sample. |
Tryptone Water Test
The Tryptone Water Test is a diagnostic procedure used to detect the presence of indole, which is an enzymatic byproduct produced by certain bacteria. Specifically, this test helps identify the presence of thermotolerant Escherichia coli (E. coli) or other thermotolerant coliforms in a water sample. The following steps outline the procedure:
- Incubation:
- Incubate the tryptone water sample at a specific temperature of 44.5 ± 0.2 degrees Celsius.
- Allow the sample to incubate for a duration of 18 to 24 hours.
- Addition of Kovacs Reagent:
- After the incubation period, add approximately 0.1 mL of Kovacs reagent to the tryptone water sample.
- Ensure thorough mixing of the reagent with the sample.
- Observation and Interpretation:
- The presence or absence of indole is determined by the observation of a color change in the Kovacs reagent.
- Positive Result:
- If a red hue appears in the Kovacs reagent and forms a film over the aqueous phase of the medium, it indicates the presence of indole.
- Additionally, positive confirmatory tests for indole, along with growth and gas generation, signify the existence of thermotolerant E. coli.
- Negative Result:
- If there is no change in color or absence of a red hue in the Kovacs reagent, it indicates the absence of indole.
- However, if there is still growth and gas production observed, it confirms the presence of thermotolerant coliforms.
The Tryptone Water Test, by detecting the presence or absence of indole, provides valuable information about the presence of specific bacteria in a water sample. Positive results for indole, growth, and gas generation are indicative of thermotolerant E. coli, while the absence of indole but the presence of growth and gas production suggests the presence of other thermotolerant coliforms. It is important to carefully interpret the results of this test in conjunction with other relevant tests and guidelines to assess the microbiological safety of the water sample.
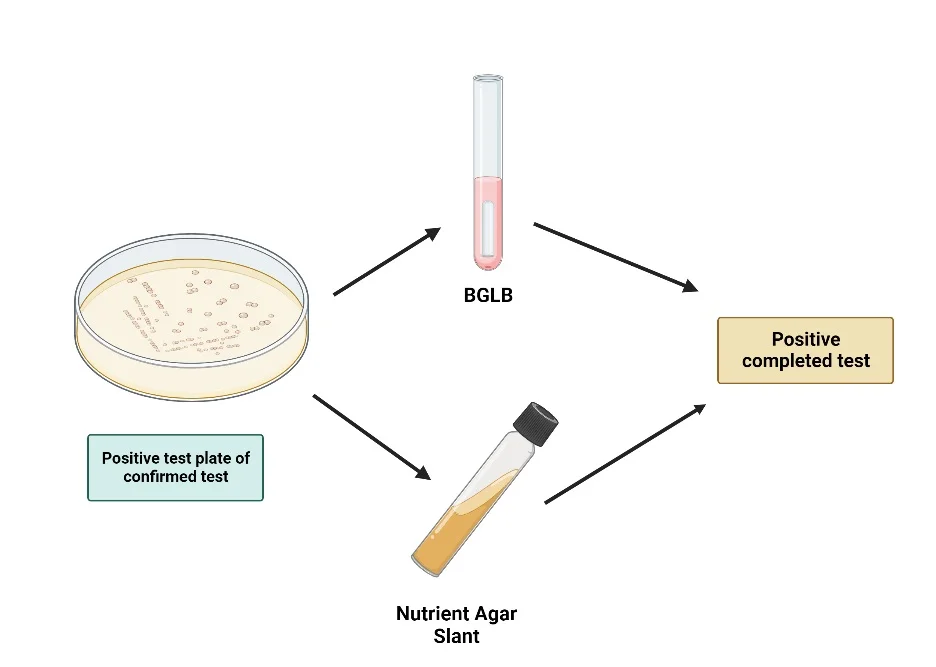
3. Completed Test of MPN Test
- The completed test in the Most Probable Number (MPN) test serves as a final confirmation for the presence of coliform bacteria, especially when results from previous tests are doubtful or need verification.
- To perform this test, a typical coliform colony from an LES Endo agar plate is selected. This colony is then inoculated into a tube of brilliant green bile broth and streaked on the surface of a nutrient agar slant. Both are incubated at 35°C for 24 hours.
- After incubation, the broth is examined for gas production, which indicates lactose fermentation by coliforms. Concurrently, a Gram stain is performed on the organisms from the nutrient agar slant. The presence of Gram-negative, non-spore-forming rods, combined with gas production in the lactose broth, confirms the presence of coliform bacteria.
- The completed test ensures the reliability of the MPN method by verifying the initial and confirmatory test results. This meticulous approach helps distinguish true coliforms from other bacteria, ensuring accurate water quality assessments.
Procedure of Completed Test
- Selection of a Typical Coliform Colony:
- Begin by identifying a typical coliform colony on the agar plate. This colony would have been previously grown and identified during the confirmatory test.
- Inoculation in Brilliant Green Bile Broth:
- Using a sterile loop, transfer the selected coliform colony into a tube containing brilliant green bile broth. Ensure that a Durham’s tube is placed inside the broth tube. The Durham’s tube will serve to capture any gas produced during fermentation, providing a visual indication of coliform activity.
- Besides the broth, streak the same colony onto the surface of a nutrient agar slant. This slant will be used for Gram staining, a technique that differentiates bacteria based on the structural characteristics of their cell walls.
- Incubation:
- Place both the brilliant green bile broth tube and the nutrient agar slant in an incubator set at 35°C.
- Allow the samples to incubate for 24 hours. This duration provides adequate time for the bacteria to grow and exhibit characteristic behaviors.
- Observation and Analysis:
- After the 24-hour incubation period, inspect the brilliant green bile broth tube. Specifically, look for the production of gas in the Durham’s tube. The presence of gas indicates fermentation, a characteristic behavior of coliform bacteria.
- Concurrently, perform a Gram stain on the organisms grown on the nutrient agar slant. This staining procedure will differentiate the bacteria based on their cell wall properties, further confirming their identity.
- Further Inoculation (if required):
- If additional validation is needed, take a loopful of culture from a tube that showed positive results in the confirmed test.
- Inoculate this culture either in BGLB medium or by streaking it onto agar slants.
- Again, incubate these test tubes for 24-48 hours at 35 degrees Celsius, observing for characteristic growth patterns and behaviors.
Completed Test Result
- Positive Result:
- Gas Production in Broth: One of the primary indicators of a positive result is the formation of gas in the brilliant green bile broth tube. This gas formation is a direct result of the fermentation process undertaken by coliform bacteria.
- Gram Staining: Besides gas production, a positive result is further confirmed by the presence of Gram-negative, non-spore-forming rods when a Gram stain is performed on the nutrient agar slant. These specific characteristics are indicative of coliform bacteria.
- Implications: A positive result in the completed test signifies the presence of coliform bacteria in the water sample. This, in turn, suggests potential fecal contamination, raising concerns about the water’s safety for consumption or use.
- Negative Result:
- Absence of Gas Production: A negative result is indicated by the lack of gas formation in the brilliant green bile broth tube. This absence suggests that the fermentation process, characteristic of coliform bacteria, did not occur.
- Gram Staining: Additionally, the absence of Gram-negative, non-spore-forming rods on the nutrient agar slant, as revealed by Gram staining, further confirms a negative result.
- Implications: A negative result in the completed test denotes the absence of coliform bacteria in the water sample. This suggests that the water is free from fecal contamination, making it safer for consumption or use.
| Test Result | Gas Formation in Brilliant Green Bile Broth | Gram Staining (NA Slant) |
|---|---|---|
| Positive | Presence | Gram-negative, non-spore-forming rods |
| Negative | Absence | Absence |
Uses/Applications of MPN Test
- Estimation in Diverse Samples:
- Soils: The MPN test is instrumental in determining microbial populations in soil samples. This is crucial for understanding soil health, fertility, and its ability to support plant growth.
- Waters: Water sources, whether natural or treated, can be assessed for microbial contamination using the MPN test. This is vital for ensuring water safety and quality.
- Agricultural Products: The test is also employed to gauge the microbial load in agricultural products, ensuring they meet safety standards before reaching consumers.
- Handling Particulate Material:
- The MPN technique stands out, especially when dealing with samples laden with particulate matter. Such samples often pose challenges for traditional plate count enumeration methods. Therefore, the MPN test offers a more effective alternative, circumventing the issues posed by particulates.
- Environmental Monitoring:
- In the realm of environmental studies, monitoring microbial populations is paramount. The MPN test has been proposed as an alternative method to trend environmental monitoring studies, providing insights into microbial dynamics in various environments.
- Counting Reluctant Bacteria:
- Not all bacteria readily form colonies on standard agar plates or membrane filters. Some might be reluctant or slow to colonize these mediums. In such scenarios, the MPN test proves invaluable. It facilitates the counting of such bacteria, as they tend to grow more willingly in liquid media.
Advantages of MPN Test
- Ease of Interpretation:
- One of the primary advantages of the MPN test is its straightforward interpretation. The results can be discerned either by direct observation of the sample or by detecting gas emission. This simplicity in interpretation ensures that even those with minimal training can effectively utilize the test.
- Dilution of Sample Toxins:
- The MPN method inherently involves dilution, which means any toxins present in the sample are diluted. This not only ensures safety during testing but also minimizes the potential impact of toxins on the test results.
- Analyzing Turbid Samples:
- The MPN test is particularly adept at analyzing samples with high turbidity. Such samples, which may include sediments, sludge, and mud, often pose challenges for other testing methods. However, the MPN test can handle these with ease, ensuring accurate results even in challenging conditions.
- Versatility in Sample Types:
- Another significant advantage of the MPN test is its ability to analyze a wide variety of water samples. This versatility is especially notable when compared to the membrane filtration method, which might not be suitable for all water types. Therefore, whether the water is clear or turbid, the MPN test remains a reliable choice.
- Applicability to Non-filterable Samples:
- Some samples, due to their composition or consistency, cannot be analyzed using membrane filtration. In such cases, the MPN test comes to the rescue, permitting the analysis of these challenging samples and ensuring that no sample is left untested.
Limitations of MPN Test
- Accuracy and Precision Concerns:
- One of the primary limitations of the MPN test is its questionable accuracy and precision. The inherent variability in the method often means that it is considered a last-resort option, especially when other counting methods are available and more appropriate.
- Labor-Intensive and Expensive:
- The MPN test can be laborious, requiring meticulous attention to detail. Besides, it demands a significant amount of materials, glassware, and incubator space, making it a more expensive option compared to some other methods.
- Large Margin of Error:
- The results obtained from the MPN test come with a relatively large margin of error. This can pose challenges, especially when precise counts are essential for decision-making or further analysis.
- Extended Duration for Results:
- To confirm the presence of coliforms, the MPN test involves a series of three tests. This sequential testing means that obtaining definitive results can be time-consuming.
- Sensitivity Issues:
- While sensitivity is often seen as a strength, in the case of the MPN test, it can sometimes lead to false results. This heightened sensitivity can occasionally detect contaminants that are not of primary concern, leading to false positives.
- Requirement of Extensive Hardware:
- The MPN method necessitates the use of multiple test tubes and a significant amount of glassware for media preparation. This not only increases the cost but also demands more storage and handling.
- Potential for False Positives:
- The probability of obtaining false positives is another limitation of the MPN test. This can lead to unnecessary interventions or further testing, increasing both time and cost.
What is the Most Probable Number (MPN) test?
The MPN test is a statistical method used to estimate the concentration of microorganisms, such as coliform bacteria, in a given sample.
When is the MPN test used?
The MPN test is commonly used in microbiology and environmental science to assess the presence or absence of specific microorganisms in samples, particularly in water and food testing.
How does the MPN test work?
The MPN test involves diluting the sample and inoculating multiple replicate tubes or wells with different dilutions. The tubes or wells are then observed for positive or negative growth, and the MPN value is determined using statistical tables.
Why is the MPN test preferred over other methods?
The MPN test is preferred in situations where quantification is required and individual colony counting is not feasible. It provides an estimate of microbial concentration based on the probability of positive growth.
What are the advantages of the MPN test?
The MPN test is relatively simple to perform, allows for quantification of microbial concentration, and provides statistical confidence in the results.
What are the limitations of the MPN test?
The MPN test provides an estimate rather than an exact count of microorganisms. It is also time-consuming compared to other rapid methods and may not be suitable for certain types of microorganisms.
What types of microorganisms can be detected using the MPN test?
The MPN test can be used to estimate the concentration of various microorganisms, including coliform bacteria, fecal indicator bacteria, and other types of bacteria that can grow in the specific growth medium.
What is the significance of the MPN value?
The MPN value indicates the probable number of microorganisms in the sample. It helps assess the quality and safety of water, food, or other tested substances.
How is the MPN value interpreted?
The MPN value is interpreted by comparing it to standard MPN tables or using statistical software to determine the range of probable microbial concentration.
Is the MPN test applicable to all types of samples?
The MPN test is commonly used for testing water samples, but it can also be applied to other types of samples, such as food, environmental swabs, or clinical specimens, depending on the target microorganism and appropriate growth medium.
References
- Williams, M. G., & Busta, F. F. (1999). TOTAL VIABLE COUNTS | Most Probable Number (MPN). Encyclopedia of Food Microbiology, 2166–2168. doi:10.1006/rwfm.1999.4000
- Rowe R, Todd R, Waide J. Microtechnique for most-probable-number analysis. Appl Environ Microbiol. 1977 Mar;33(3):675-80. doi: 10.1128/aem.33.3.675-680.1977. PMID: 16345226; PMCID: PMC170744.
- Sui Sien, Leong & Ismail, Johan & Denil, N.A. & Sarbini, Shahrul & Wasli, Wafri & Lingoh, Arlene. (2018). Microbiological and Physicochemical Water Quality Assessments of River Water in an Industrial Region of the Northwest Coast of Borneo. Water. 10. 1648. 10.3390/w10111648.
- Chandrapati, S., & Williams, M. G. (2014). TOTAL VIABLE COUNTS | Most Probable Number (MPN). Encyclopedia of Food Microbiology, 621–624. doi:10.1016/b978-0-12-384730-0.00333-5
- http://www.microbiologynetwork.com/doc/sutton.jvt_.16.3.pdf
- http://egyankosh.ac.in/bitstream/123456789/31151/1/Exp-15.pdf
- http://faculty.collin.edu/dcain/ccccdmicro/most_probable_number_presumptive.htm
- https://vet.uga.edu/lab-test/bacteriology-most-probable-number-mpn/
- http://jkp.poltekkes-mataram.ac.id/index.php/home/article/view/17
- https://vet.uga.edu/lab-test/bacteriology-most-probable-number-mpn/
- https://microbenotes.com/water-quality-analysis-by-most-probable-number-mpn/
- https://biologyreader.com/most-probable-number-method.html
- https://www.onlinebiologynotes.com/most-probable-number-mpn-method-for-counting-coliform/
- https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions
- https://support.hach.com/myhach/s/login/?language=en_US&ec=302&inst=3q&startURL=httpssupport.hach.commyhachappanswersanswer_viewa_id1020234microbiology-guide3A-most-probable-number-28mpn29-method-
- https://en.wikipedia.org/wiki/Most_probable_number
- https://www.integra-biosciences.com/global/en/applications/rapid-and-precise-dilutions-most-probable-number-test-procedure-dose-it
- http://www.corrosionpedia.com/definition/794/most-probable-number-mpn
- https://www.slideshare.net/SamsuDeen12/most-probable-number-or-multiple-tube-fermentation-technique
- https://www.studocu.com/in/login
- https://microbeonline.com/probable-number-mpn-test-principle-procedure-results/