What is Modified Thayer Martin Agar?
Modified Thayer Martin agar (MTM agar) is a specialized culture medium used for the isolation and identification of Neisseria gonorrhoeae, the bacterium responsible for the sexually transmitted infection gonorrhea. It is derived from the original Thayer-Martin agar, with modifications made to enhance its selectivity and suppress the growth of contaminants.
The primary purpose of MTM agar is to selectively isolate Neisseria gonorrhoeae from clinical specimens that may contain a mixture of different bacteria and fungi. These specimens are typically collected from urogenital sites. MTM agar contains a base similar to that of chocolate agar, which provides the necessary nutrients for the growth of Neisseria strains.
To inhibit the growth of other microorganisms present in the specimen, MTM agar incorporates several antimicrobial agents. These include:
- Vancomycin: Vancomycin inhibits the growth of gram-positive bacteria. By including this antibiotic in the medium, it prevents the overgrowth of unwanted gram-positive organisms that may be present in the specimen.
- Colistin: Colistin is effective against gram-negative bacteria, including commensal Neisseria species. Its presence in MTM agar helps suppress the growth of non-pathogenic Neisseria strains that may be present alongside N. gonorrhoeae.
- Nystatin: Nystatin is an antifungal agent. Its inclusion in the medium prevents the growth of yeast and fungal contaminants that could interfere with the isolation of Neisseria gonorrhoeae.
- Trimethoprim lactate: Trimethoprim inhibits the swarming behavior of Proteus species. By adding trimethoprim lactate to the medium, the swarming of Proteus spp. is suppressed, enabling the selective isolation of N. gonorrhoeae.
The combination of these antimicrobial agents in MTM agar creates an environment that supports the growth of N. gonorrhoeae while inhibiting the growth of most other gram-negative diplococci, gram-negative bacilli, gram-positive organisms, and yeast.
The development of Modified Thayer Martin agar originated from the work of Thayer and Martin, who improved the formulation of Chocolate Agar by adding antimicrobics, including vancomycin, colistin, and nystatin. This modification aimed to minimize overgrowth by contaminants, suppress the growth of non-pathogenic Neisseria species, and enhance the growth of pathogenic Neisseria strains.
Later, Martin and Lester further modified the medium by adding trimethoprim to enhance its selectivity. The addition of trimethoprim lactate proved to be valuable in suppressing the swarming behavior of Proteus species, a characteristic that can interfere with the isolation of N. gonorrhoeae. The resulting medium was named Modified Thayer Martin agar, which serves as a selective and enriched medium for the isolation and cultivation of Neisseria species from mixed flora, while suppressing the growth of unwanted microorganisms.
In summary, Modified Thayer Martin agar is a specialized culture medium designed for the selective isolation of Neisseria gonorrhoeae from specimens containing a mixed flora. It incorporates specific antimicrobial agents to inhibit the growth of unwanted organisms, creating an environment conducive to the growth of pathogenic Neisseria strains.
Composition of Modified Thayer Martin Agar
The composition of Modified Thayer Martin agar includes the following ingredients:
- Casein peptone: 7.5g
- Meat peptone: 7.5g
- Corn Starch: 1.0g
- Dipotassium Phosphate: 4.0g
- Monopotassium Phosphate: 1.0g
- Sodium Chloride: 5.0g
- Agar: 12.0g
- Hemoglobin solution: 10.0g
- IsovitaleX enrichment: 10.0ml
- Vancomycin: 300μg
- Colistin: 750μg
- Nystatin: 12,500 U
- Trimethoprim: 5.0mg
- Demineralized water: 1000.0ml
- Final pH = 7.2 ± 0.2 at 25°C
IsovitaleX enrichment, which is added to Modified Thayer Martin agar, consists of the following components:
- Dextrose: 100.0g
- Vitamin B12: 0.01g
- L-Glutamine: 10.0g
- Thiamine HCl: 0.003g
- L-Cystine: 1.1g
- Adenine: 1.0g
- p-aminobenzoic acid: 13.0g
- Cocarboxylase: 0.1g
- Cystine HCL: 25.9g
- Guanine HCL: 0.03g
- NAD: 0.25g
- Ferric nitrate: 0.02g
- Demineralized water: 1000.0ml
These ingredients work together to create a selective and enriched medium for the isolation and cultivation of Neisseria gonorrhoeae while inhibiting the growth of other unwanted microorganisms. The final pH of the medium is maintained at 7.2 ± 0.2 at 25°C.
It’s important to note that the provided composition is a general reference and specific formulations may vary slightly depending on the manufacturer or laboratory protocols.
Purpose
Modified Thayer Martin Medium Base utilized for the selective isolation and the enumeration of Neisseria species, particularly Neisseria gonorrhoeae
Principle of Modified Thayer Martin Agar
The principle of Modified Thayer Martin agar (MTM agar) revolves around its selective and enriched composition, which facilitates the isolation and cultivation of pathogenic Neisseria gonorrhoeae while inhibiting the growth of other microorganisms.
The agar base used in MTM agar is derived from Chocolate II Agar, known as GC II base. This base consists of casein and meat peptones, which provide nitrogenous nutrients for bacterial growth. It also includes a phosphate buffer that helps maintain the pH of the medium and corn starch, which neutralizes toxic fatty acids that may be present in the agar. The addition of dextrose in MTM agar enhances the growth of gonococci, promoting their proliferation.
The inclusion of bovine hemoglobin in the medium serves as a source of X factor (hemin). Hemin is an essential growth factor for Neisseria species and is required for their optimal growth. By providing hemin through the hemoglobin present in the medium, MTM agar supports the growth of pathogenic Neisseria strains.
To further enhance the growth of pathogenic Neisseria, MTM agar incorporates IsovitaleX enrichment. This enrichment supplement contains various components that provide the V factor (nicotinamide adenine dinucleotide, NAD), vitamins, amino acids, coenzymes, dextrose, ferric ion, and other factors necessary for the improved growth of Neisseria species.
MTM agar also contains antimicrobial agents to suppress the growth of normal flora and unwanted microorganisms. Vancomycin, which primarily targets gram-positive bacteria, helps inhibit the growth of these organisms, reducing their interference with the isolation of N. gonorrhoeae. Colistin, on the other hand, inhibits the growth of gram-negative bacteria, including Pseudomonas species.
Nystatin, an antifungal agent, is included in MTM agar to prevent the growth of Candida albicans, a common yeast species that can be present in clinical specimens. The presence of nystatin helps maintain the selectivity of the medium by suppressing fungal contaminants.
Additionally, MTM agar contains trimethoprim lactate, which acts as an inhibitor against the swarming behavior of Proteus species. By restraining the swarming of Proteus, this component further contributes to the selectivity of MTM agar, allowing for the isolation of N. gonorrhoeae without interference from Proteus spp.
In summary, the principle of Modified Thayer Martin agar lies in its selective and enriched composition. It incorporates specific nutrients, growth factors, and antimicrobial agents to create an environment that promotes the growth of pathogenic Neisseria strains while inhibiting the growth of other microorganisms, thereby facilitating the isolation and identification of Neisseria gonorrhoeae.
Preparation of Modified Thayer Martin Agar
The preparation of Modified Thayer Martin agar involves several steps to ensure the proper combination of ingredients and sterility. Here is a step-by-step guide to preparing the medium:
- Preparation of the GC Agar base:
- Add the components of the GC medium base into distilled water.
- Bring the volume to 730.0ml and mix thoroughly.
- Gently heat the mixture until it reaches boiling point.
- Autoclave the mixture at 121°C for 15 minutes.
- Allow the GC Agar base to cool to a temperature of 45-50°C.
- Preparation of Hemoglobin solution:
- Add the desired amount of hemoglobin to distilled water.
- Bring the volume to 250.0ml and mix thoroughly.
- Autoclave the hemoglobin solution at 121°C for 15 minutes.
- Allow the solution to cool to a temperature of 45-50°C.
- Preparation of IsoVitaleX enrichment (10ml):
- Add the components of IsoVitaleX enrichment to distilled water.
- Bring the volume to 10.0ml and mix thoroughly.
- Filter sterilize the solution to ensure sterility.
- Preparation of VCNT antibiotic solution (10ml):
- Add the components of the VCNT antibiotic solution to distilled water.
- Bring the volume to 10.0ml and mix thoroughly.
- Filter sterilize the solution to ensure sterility.
- Preparation of the medium:
- Take the cooled, sterile GC agar base (730.0ml) and transfer it to an aseptic environment.
- Aseptically add the sterile hemoglobin solution (250.0ml) to the GC agar base.
- Add 10.0ml of the IsoVitaleX enrichment and 10.0ml of the VCNT antibiotic solution to the mixture.
- Thoroughly mix all the components to ensure uniform distribution.
- Pour the mixture into sterile Petri dishes or distribute it into sterile tubes.
After pouring or distributing the medium, allow it to solidify and then label and store the plates or tubes in appropriate conditions. The prepared Modified Thayer Martin agar is now ready to be used for the isolation and cultivation of Neisseria gonorrhoeae.
Physical Properties of Modified Thayer Martin Agar
- Appearance: Cream to yellow homogeneous free flowing powder
- Gelling: Firm, comparable with 1.3% Agar gel.
- Colour and Clarity of prepared medium: The Basal Medium is a yellow transparent to slightly opalescent gel. Following the addition of haemoglobin or the sterile lysed blood, and any other ingredients chocolate colored opaque gel forms inside Petri plates.
- Reaction: Reaction of 4.2% w/v aqueous solution at 25°C. pH : 7.0±0.2
- pH: 6.80-7.20
Cultural Response
M413: Cultural traits discovered with added sterilelysed blood/Haemoglobin solutions (FD022), Vitamino Growth Supplement (FD025) and V.C.N. Supplement (FD023)/V.C.N.T. Supplement (FD024) after an incubation of 35-37°C for 18 to 48 hours
Result Interpretation of Modified Thayer Martin Agar
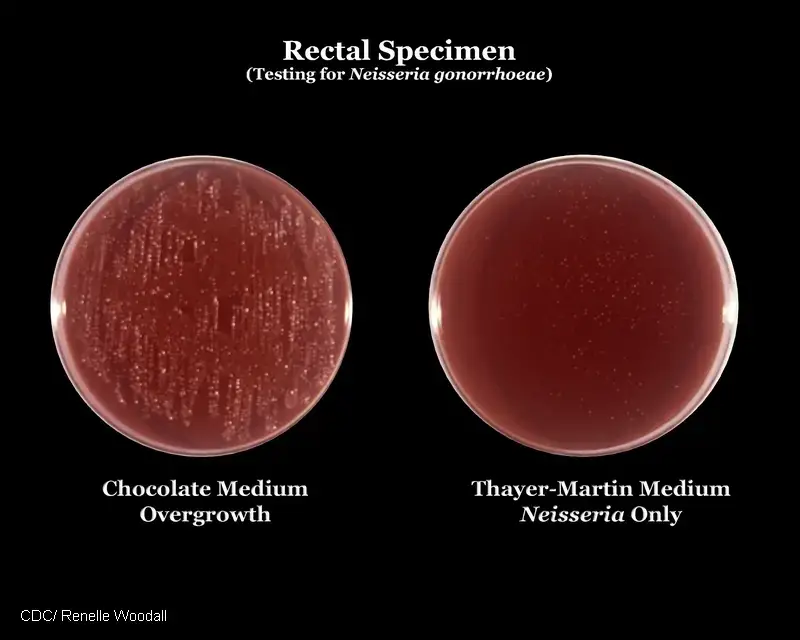
The interpretation of results on Modified Thayer Martin agar involves the observation of colonial morphology and the presence or absence of specific microorganisms. Here is a guide to interpreting the results:
- Neisseria gonorrhoeae:
- Typical colonial morphology: Small, grayish-white to colorless, mucoid colonies.
- Consistency: Smooth.
- Margins: Well-defined.
- Size: Typically 0.5 – 1.0 mm in diameter.
- Neisseria meningitidis:
- Typical colonial morphology: Medium to large colonies.
- Color: Blue-gray.
- Consistency: Mucoid.
The colonies of N. meningitidis should appear as large, round, smooth, convex, colorless-to-grey, and opaque on Modified Thayer Martin agar. There should be no discoloration of the medium around the colonies.
It is important to note that these observations are specific to Neisseria species and may vary slightly depending on the strain and other factors. Confirmatory tests, such as Gram staining and biochemical tests, should be performed for accurate identification of Neisseria strains.
Additionally, after 48 hours of incubation, the sterility test plate should remain clear, indicating the absence of any microbial growth. This is important to verify the sterility of the medium itself.
Interpretation of the results on Modified Thayer Martin agar should be done by comparing the observed colonial morphology and characteristics with the expected features of Neisseria species.
Culture and Isolation
To culture and isolate Neisseria gonorrhoeae, the following steps are typically followed:
- Specimen Collection:
- Urethral or endocervical specimens are collected using swabs with plastic or wire shafts and tips made of rayon, Dacron, or calcium alginate.
- The swab is used to collect the specimen from the appropriate site.
- Inoculation of Culture Plates:
- The collected specimen is directly inoculated onto culture plates. The swab is streaked or rolled onto the surface of the culture medium.
- Modified Thayer Martin agar is commonly used as the selective and enriched medium for the isolation of N. gonorrhoeae.
- Care should be taken to ensure proper inoculation of the culture medium.
- Incubation:
- The inoculated culture plates are promptly placed into a CO2-enriched environment. CO2 concentration in the range of 3% to 10% is commonly used.
- The plates are incubated at a temperature of 35°C to 37°C, which provides an optimal growth environment for N. gonorrhoeae.
- Colony Characteristics:
- After incubation, the culture plates are examined for colony growth and characteristics.
- Neisseria gonorrhoeae colonies typically appear as small, opaque, grayish-white to colorless, raised, glistening, and smooth colonies.
- These colonies are often described as having a mucoid or moist appearance.
It is important to note that while the appearance described above is typical for N. gonorrhoeae, confirmatory tests such as Gram staining and biochemical tests should be performed to definitively identify the bacteria.
The culture and isolation process allows for the growth and multiplication of N. gonorrhoeae on the selective medium, facilitating its subsequent identification and further testing for antimicrobial susceptibility, if required.
Quality control
Quality control of Modified Thayer Martin agar (MTM agar) ensures the reliability and performance of the medium for the isolation and cultivation of pathogenic Neisseria species. Here are the key aspects of quality control for MTM agar:
- Appearance: The MTM agar should have a cream to yellow homogeneous free-flowing powder appearance.
- Gelling: The gelling property of the medium should be firm and comparable to a 1.3% agar gel.
- Colour and Clarity of Prepared Medium: The basal medium should form a yellow-colored, clear to slightly opalescent gel. After the addition of hemoglobin or sterile lysed blood and supplements, the medium should form a chocolate-colored, opaque gel in Petri plates.
- Reaction: The pH of a 4.2% w/v aqueous solution of MTM agar at 25°C should be 7.0 ± 0.2.
- pH: The pH range of the prepared medium should be 6.80-7.20.
- Cultural Response: The cultural characteristics of selected microorganisms should be observed when inoculated on MTM agar with the addition of sterile lysed blood/hemoglobin solution, Vitamino Growth Supplement, and V.C.N. or V.C.N.T. Supplement after incubation at 35-37°C for 18-48 hours.
- Escherichia coli ATCC 25922: The inoculum of E. coli should be inhibited, resulting in no growth observed.
- Neisseria gonorrhoeae ATCC 19424: Growth recovery should be good to luxuriant, with small, grayish-white to colorless, mucoid colonies comprising at least 50% of the growth.
- Neisseria meningitidis ATCC 13090: Growth recovery should be good to luxuriant, with medium to large, blue-gray, mucoid colonies comprising at least 50% of the growth.
- Proteus mirabilis ATCC 25933: The inoculum of P. mirabilis should be inhibited, resulting in no growth observed.
By performing these quality control tests, laboratories can verify that the MTM agar is suitable for its intended purpose, ensuring accurate and reliable results during the isolation and identification of pathogenic Neisseria strains.
Uses of Modified Thayer Martin Agar
Modified Thayer Martin agar (MTM agar) has several important uses in microbiology, particularly in the isolation and cultivation of pathogenic Neisseria species. Here are the primary uses of MTM agar:
- Isolation of Neisseria species: MTM agar is specifically designed to selectively isolate and cultivate pathogenic Neisseria species, such as Neisseria gonorrhoeae and Neisseria meningitidis. These bacteria are responsible for the sexually transmitted infection gonorrhea and meningococcal meningitis, respectively. MTM agar provides a suitable environment that promotes the growth of these pathogenic Neisseria strains while suppressing the growth of other microorganisms present in clinical specimens.
- Selective medium: MTM agar acts as a selective medium for the isolation of Neisseria species. It contains antimicrobial agents, such as vancomycin, colistin, and nystatin, which inhibit the growth of gram-positive bacteria, gram-negative bacteria (including commensal Neisseria species), and yeast/fungal contaminants, respectively. By suppressing the growth of unwanted organisms, MTM agar enables the isolation and identification of pathogenic Neisseria strains from specimens that may contain a mixed flora of bacteria and fungi.
- Cultivation of Neisseria species: In addition to isolation, MTM agar provides a rich and nutrient-filled medium that supports the growth and cultivation of Neisseria species. The agar base, along with the inclusion of casein and meat peptones, provides the necessary nitrogenous nutrients for bacterial growth. Hemoglobin and IsoVitaleX enrichment supply essential growth factors, such as X factor (hemin) and V factor (nicotinamide adenine dinucleotide, NAD), respectively, which are required for the optimal growth of Neisseria species.
- Diagnostic purposes: MTM agar is widely used in clinical microbiology laboratories for the diagnosis of Neisseria infections. By isolating and identifying pathogenic Neisseria strains, the agar helps in the confirmation of gonorrhea and meningococcal infections. Further testing, such as antimicrobial susceptibility testing, can be performed on the isolated bacteria to guide appropriate treatment decisions.
Limitations of Modified Thayer Martin Agar
Modified Thayer Martin agar (MTM agar) has certain limitations that should be taken into consideration. Here are the key limitations associated with its use:
- Confirmatory testing required: While MTM agar provides selective conditions for the growth of pathogenic Neisseria species, additional biochemical and serological testing are recommended for the final confirmation of the isolated bacteria. These confirmatory tests help differentiate Neisseria species from other closely related bacteria and ensure accurate identification.
- Optimal CO2 concentration: MTM agar requires incubation in a CO2-enriched environment, typically ranging from 3% to 7% CO2. Higher concentrations of CO2 can be inhibitory to certain strains of Neisseria, impacting their growth and detection. Therefore, it is important to maintain the appropriate CO2 concentration during incubation.
- Inhibition of other pathogenic bacteria: The selective components of MTM agar designed to suppress unwanted microorganisms, such as vancomycin, colistin, and nystatin, may also inhibit the growth of other pathogenic bacteria. For example, Haemophilus species can be inhibited by the selective nature of MTM agar. Therefore, alternative media or specific tests may be required for the detection and isolation of these bacteria.
- Inhibited strains of N. gonorrhoeae: Some strains of N. gonorrhoeae have been reported to be inhibited by the components of the V-C-N inhibitor and trimethoprim lactate present in MTM agar. This can result in the failure to isolate these particular strains using this medium.
- Occasional recovery of non-pathogenic Neisseria: While the selective components of MTM agar generally suppress non-pathogenic “saprophytic” Neisseria, there have been occasional reports of the recovery of N. lactamica, a non-pathogenic Neisseria species, on Thayer-Martin selective agar. This highlights the need for careful interpretation and further testing to distinguish between pathogenic and non-pathogenic Neisseria strains.
- Growth of Capnocytophaga species: Some strains of Capnocytophaga species, particularly when inoculated with oropharyngeal specimens, may grow on MTM agar or similar selective media. These bacteria have the potential to grow despite the selective components of the medium, leading to potential false-positive results or interference with the isolation of pathogenic Neisseria.
Awareness of these limitations helps in the appropriate interpretation of results and ensures that additional tests or alternative media are used when necessary to overcome these challenges and achieve accurate identification and diagnosis.
FAQ
What is Modified Thayer Martin agar used for?
Modified Thayer Martin agar is used for the selective isolation and cultivation of pathogenic Neisseria species, particularly Neisseria gonorrhoeae, from specimens containing mixed flora of bacteria and fungi.
What are the key components of Modified Thayer Martin agar?
Modified Thayer Martin agar contains a base similar to chocolate agar, hemoglobin for X factor (hemin), IsoVitaleX enrichment for V factor (NAD), and antimicrobial agents such as vancomycin, colistin, nystatin, and trimethoprim lactate.
How does Modified Thayer Martin agar selectively isolate Neisseria species?
Modified Thayer Martin agar suppresses the growth of most gram-negative diplococci, gram-negative bacilli, gram-positive organisms, and yeast, while allowing the growth of pathogenic Neisseria strains.
What is the appearance of Neisseria gonorrhoeae colonies on Modified Thayer Martin agar?
Neisseria gonorrhoeae colonies appear as small, grayish-white to colorless, mucoid colonies with a smooth consistency and defined margins, typically measuring 0.5-1.0 mm in diameter.
Can other pathogenic bacteria grow on Modified Thayer Martin agar?
The selective nature of Modified Thayer Martin agar can inhibit the growth of other pathogenic bacteria, such as Haemophilus species. Alternative media or specific tests may be required for their detection and isolation.
What are the limitations of Modified Thayer Martin agar?
Some strains of N. gonorrhoeae may be inhibited by the components of the medium. Additionally, non-pathogenic Neisseria species and certain Capnocytophaga species may occasionally grow on the medium, requiring careful interpretation and further testing.
What is the recommended incubation condition for Modified Thayer Martin agar?
Modified Thayer Martin agar plates should be incubated in a CO2-enriched environment (3%-10%) at a temperature of 35-37°C.
What confirmatory tests are recommended when using Modified Thayer Martin agar?
Additional biochemical and serological tests are recommended for the final confirmation of Neisseria species isolated on Modified Thayer Martin agar.
How should Modified Thayer Martin agar be stored?
Modified Thayer Martin agar should be stored in a dry place at room temperature. Prepared plates should be stored upside down to prevent condensation.
What are the quality control parameters for Modified Thayer Martin agar?
Quality control of Modified Thayer Martin agar includes checking its appearance, gelling property, color and clarity of prepared medium, pH, and cultural response with specified microorganisms, such as Neisseria gonorrhoeae, Neisseria meningitidis, Escherichia coli, and Proteus mirabilis.
References
- https://hardydiagnostics.com/media/assets/product/documents/ChocolateAgar.pdf
- http://www.dalynn.com/dyn/ck_assets/files/tech/PT36.pdf
- https://microbeonline.com/thayer-martin-agar-composition-preparation-uses-colony-characteristics/
- https://legacy.bd.com/europe/regulatory/Assets/IFU/US/L007392(09)(0907).pdf
- https://exodocientifica.com.br/_technical-data/M413.pdf
- https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU1880.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.