Pectin is a complicated polysaccharide that is mostly found in the cell walls of plants, notably fruits. It is a structural heteropolysaccharide made up mostly of units of galacturonic acid.
Pectin is a glue that holds plant cells together, making the plant tissue strong and stable. In the culinary sector, it is often used as a thickening, gelling agent, and stabilizer, especially in jellies and jams.
Henri Braconnot originally isolated pectin from plants in 1825. In the late 1800s and early 1900s, people found out that it could gel, which made it more important. Now, it is mostly derived from citrus peels and apple pomace.
Knowing how pectin works and what its structure is helps us understand what it does in plants and in industry.
So, to sum up, pectin is a plant polysaccharide that helps cells stick together and is used a lot in food technology since it can make gels.
What is Pectin?
Pectin is a type of polysaccharide that is present in the cell walls of plants. It is especially common in fruits like apples, citrus, and quinces.
It is mostly made up of galacturonic acid units that are joined together by α-1,4-glycosidic linkages to make a backbone called homogalacturonan.
Pectin is very important for plant cells to stick together and keep their shape, especially in the middle lamella, where it holds cells together.
During the ripening of fruit, enzymes like pectinase and pectinesterase break down pectin, which makes the fruit tissue softer.
Pectin is taken from citrus peels and apple pomace for commercial usage. It is widely employed as a gelling ingredient in jams and jellies.
There are two primary kinds of commercial pectin: high methoxyl (HM) pectin, which needs a lot of sugar and acid to gel, and low methoxyl (LM) pectin, which gels when calcium ions are present and is used in goods that don’t have a lot of sugar or don’t have any sugar at all.
Pectin is also used in the pharmaceutical business since it can make gels and is a good source of dietary fiber.
Pectin is a soluble fiber that can help decrease cholesterol levels in the blood and make the digestive system work better.
Pectin’s molecular structure lets it trap water and generate gels. This feature is used in many cooking and industrial applications.
Properties of pectin
- Pectin dissolves in hot water but not in alcohol or chemical solvents.
- Formation of gels: high methoxyl pectin makes gels under acidic conditions with a lot of sugar, while low methoxyl pectin makes gels when calcium ions are present.
- The degree of esterification has an effect on how well it gels and how well it dissolves.
- The molecular weight changes, which affects the viscosity and strength of the gel.
- Viscosity: Pectin solutions have a high viscosity that depends on the concentration and size of the molecules.
- Stability: stable at acidic pH but breaks down in alkaline circumstances and by enzymes like pectinases.
- Thermal behaviour: pectin gels form at moderate temperatures and melt when heated.
- Natural polymer that is not harmful and breaks down in the environment
- It acts as dietary fibre since it can’t be digested in the human digestive tract.
- The chemical makeup includes galacturonic acid units that are linked by α-(1→4) bonds and have different levels of methyl esterification.
Tyeps of pectin
There are several types of pectin, which differ in their chemical composition and gelling properties. Here are some of the main types:
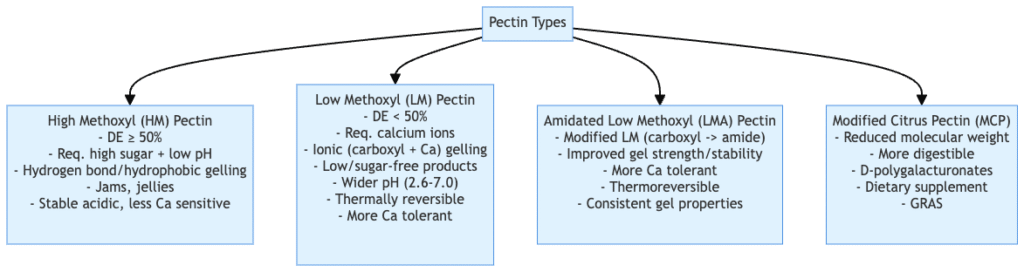
- High Methoxyl (HM) Pectin:
- Degree of esterification ≥ 50%.
- Requires high sugar content and low pH for gel formation.
- Forms gels through hydrogen bonds and hydrophobic interactions.
- Commonly used in traditional jams, jellies, and confectioneries.
- Gelation is influenced by sugar concentration and pH.
- Gels are stable under acidic conditions.
- Less sensitive to calcium ion concentration.
- Low Methoxyl (LM) Pectin:
- Degree of esterification < 50%.
- Requires calcium ions for gel formation.
- Forms gels through ionic interactions between carboxyl groups and calcium ions.
- Suitable for low-sugar or sugar-free products.
- Gels can form at a wider pH range (2.6–7.0).
- Gels are thermally reversible and can melt upon heating.
- More tolerant to variations in calcium ion concentration.
- Amidated Low Methoxyl Pectin (LMA):
- Modified form of LM pectin where some carboxyl groups are converted to amide groups.
- Offers improved gel strength and stability.
- More tolerant to variations in calcium ion concentration.
- Gels are thermoreversible, allowing for melting and re-gelling upon heating.
- Suitable for applications requiring consistent gel properties.
- Modified Citrus Pectin (MCP):
- Derived from citrus peels and modified to reduce molecular weight.
- More digestible form of pectin.
- Composed predominantly of D-polygalacturonates.
- Used as a dietary supplement for its potential health benefits.
- Generally recognized as safe for consumption.
Importance of pectin substances
- Pectin is a type of soluble dietary fibre that helps the digestive system work better.
- makes it harder for the stomach and intestines to break down, which makes poop bigger and slows down stomach emptying.
- acts as a prebiotic by feeding good gut bacteria and making short-chain fatty acids that are good for colon health.
- It helps lower cholesterol levels in the blood.
- binds bile acids, stops micelle production, and lowers cholesterol absorption
- Clinical research demonstrates that 6 g/day decreases LDL and the risk of coronary heart disease.
- It helps keep blood sugar levels stable.
- makes a thick gel that slows down the absorption of carbohydrates and makes insulin work better
- Pectin has both antioxidant and anti-inflammatory properties.
- removes free radicals, which could lower oxidative stress
- could help keep allergies and long-term inflammatory disorders from happening
- It fights bacteria and viruses.
- stops organisms including E. coli, Salmonella, and Staph. aureus from growing
- Importance of industry in food technology
- important for thickening, gelling, and stabilising jams, jellies, drinks, dairy items, and baked goods
- improves texture, stops syneresis, and makes things last longer
- Low-esterified versions keep acidic milk drinks and low-sugar foods stable.
- Uses in medicine and pharmaceuticals
- utilised in methods for delivering drugs (controlled release, gastro-retentive, colon-specific)
- makes edible films and wound treatments; works as a demulcent in lozenges
- shows promise for uses in protecting against radiation and removing heavy metals
- Applications in the environment and other areas
- biodegradable polymer that can be used for packaging, making foam, and cleaning up wastewater
- Pectinase enzymes help with making textiles, clarifying juice, and making wine.
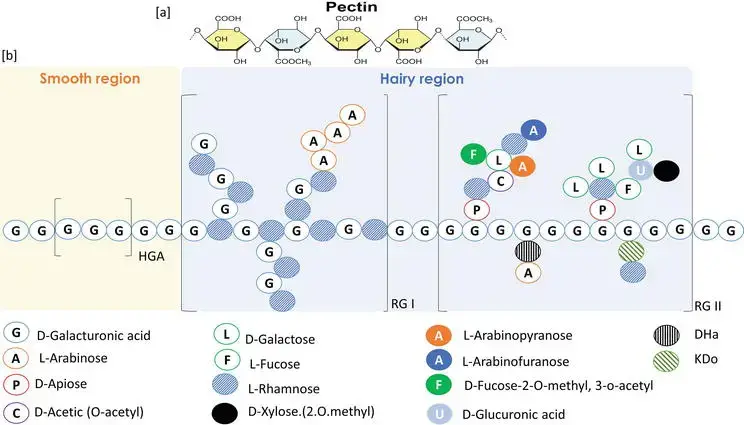
Structure of pectin
- Pectin is a complex polysaccharide predominantly consisting of D-galacturonic acid (GalA) units connected by α-(1→4) glycosidic linkages.
- The primary structure of pectin is referred to as homogalacturonan (HG), comprising linear sequences of GalA residues.
- About 65–74% of the carboxyl groups in GalA residues are esterified with methanol, resulting in methyl esters; the remaining groups exist as free acids or salts, affecting the degree of esterification (DE) and gelling characteristics.
- Pectin comprises neutral sugar side chains, such as rhamnose, galactose, arabinose, xylose, and apiose, which extend from the HG backbone, forming “hairy regions” that enhance its structural complexity.
- The side chains constitute components of intricate structures like rhamnogalacturonan I (RG-I), rhamnogalacturonan II (RG-II), and xylogalacturonan (XGA), which are substituted galacturonans exhibiting diverse levels of branching and complexity.
- RG-I consists of a recurring disaccharide unit of α-D-GalA-(1→2)-α-L-Rha-(1→4)-, accompanied by supplementary neutral sugar side chains.
- RG-II is a complex, highly branched structure consisting of 12 distinct monosaccharides, including D-galacturonic acid, and is cross-linked by borate ions, which enhances the stiffness of the plant cell wall.
- XGA is a galacturonan backbone modified with xylose units, contributing to cell wall construction and function.
- The molecular weight of pectin fluctuates based on its origin and extraction technique, spanning from roughly 28 kDa to exceeding 750 kDa.
- The structural diversity of pectin enables it to fulfil multiple roles in plants, such as imparting mechanical strength to cell walls and facilitating cell adhesion.
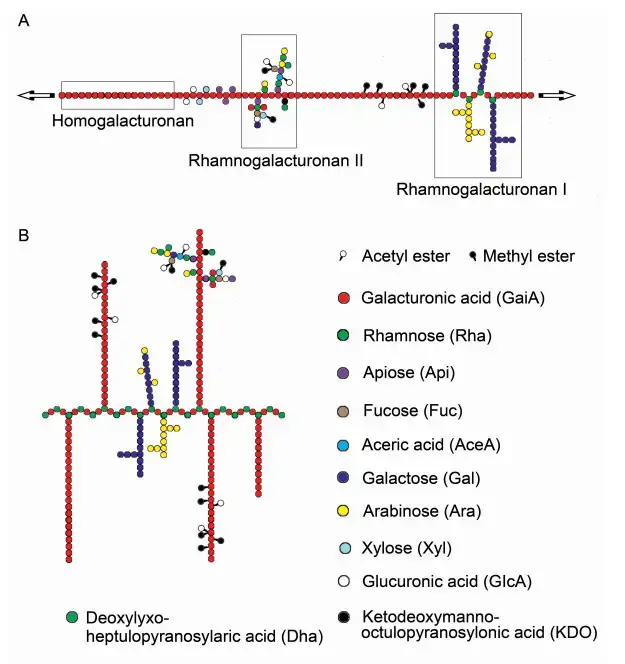
Pectin Substrates
Pectinase enzymes hydrolyze pectin, a polysaccharide prevalent in plant cell walls, via hydrolysis, trans-elimination, and de-esterification processes.
The principal substrates for pectinases comprise:
- Polygalacturonic acid- The fundamental structure of pectin, composed of α-(1→4)-linked D-galacturonic acid residues.
- Citrous pectin – Extracted from citrous fruits, abundant in galacturonic acid residues.
- Pectate- The de-esterified variant of pectin, produced by the enzymatic activity of pectinesterase.
- Protopectin- An insoluble precursor of pectin present in unripe fruits, which is transformed into soluble pectin by protopectinase.
- Arabinogalactan- A polysaccharide composed of arabinose and galactose units, occasionally found in pectin formulations.
- Xylan– is a hemicellulosic polysaccharide consisting of xylose units, which may undergo co-degradation with pectin in certain substrates.
- Galactose- A monosaccharide that may be liberated during the catabolism of pectin.
- Arabinose– A monosaccharide that may be liberated after the breakdown of pectin.
- Rhamnose -A monosaccharide that may be liberated after the breakdown of pectin.
- Xylose: A monosaccharide that may be liberated during the decomposition of pectin.
Pectinase enzymes are synthesised by diverse microorganisms, including fungi, bacteria, and yeasts, and are employed in numerous industrial applications, such as fruit juice clarifying, wine making, and textile processing.
What are Pectinases?
Pectinases are enzymes that facilitate the degradation of pectin, a complex polysaccharide present in plant cell walls, by hydrolysis, trans-elimination, and de-esterification processes.
These enzymes are synthesised by a variety of species, including fungus, bacteria, yeasts, insects, and plants. Fungi like Aspergillus niger and Penicillium occitanis are important industrial producers of pectinases.
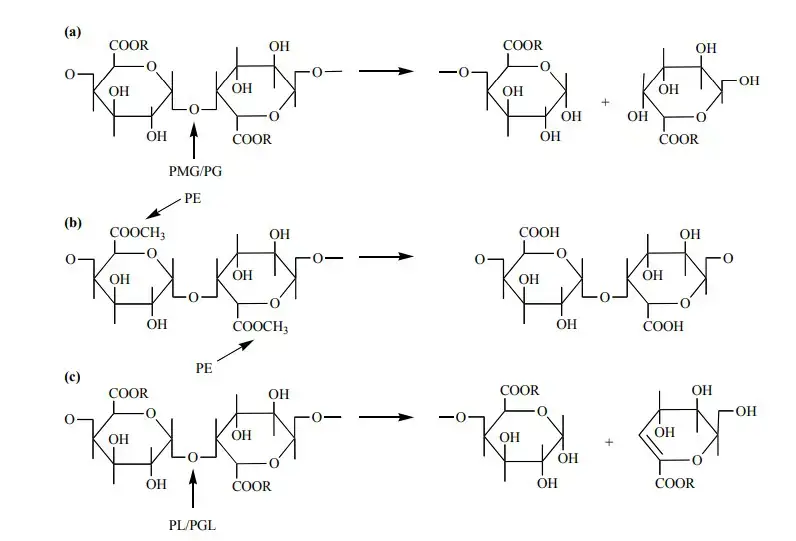
Pectinases are categorised into three primary kinds according to their method of action:
- Polygalacturonase (PG): Catalyses the hydrolysis of α-1,4-glycosidic linkages between galacturonic acid units in pectin, yielding smaller oligosaccharides.
- Pectinesterase (PE): Catalyses the removal of methyl esters from pectin, transforming it into pectate and methanol.
- Pectin Lyase (PL): Disassembles glycosidic linkages in pectin via a β-elimination process, yielding unsaturated compounds.
The activity of pectinases is affected by pH, temperature, and the presence of metal ions. The ideal circumstances differ based on the particular type of pectinase and its origin.
Pectinases are extensively utilised throughout multiple industries:
- Fruit Juice and Wine Production: Facilitate juice extraction and clarifying by degrading pectin, which may induce turbidity.
- The textile industry utilises the retting method to eliminate pectin from plant fibres, hence aiding in textile manufacture.
- Paper Industry: Utilised to augment paper quality by boosting the retention of fines and filler particles.
- Wastewater Treatment: Facilitate the decomposition of pectin-rich waste, contributing to the treatment process.
The commercial manufacturing of pectinases entails fermentation techniques employing microorganisms, succeeded by extraction and purification to yield enzyme preparations for industrial uses.
Microorganisms involved in pectin degradation – pectinolytic microorganisms
Bacteria
- Erwinia spp.
- Erwinia carotovora and Erwinia chrysanthemi are prominent pectinolytic bacteria.
- Secrete virulence factors like pectate lyases and polygalacturonases.
- Degrade pectin in plant cell walls, causing soft rot and tissue maceration.
- Utilize pectin through extracellular, periplasmic, and cytoplasmic pathways .
- Pseudomonas spp.
- Pseudomonas species are involved in pectin degradation.
- Contribute to the breakdown of pectin during processes like tobacco fermentation .
- Acidobacterium capsulatum
- Acidophilic bacterium capable of degrading pectin.
- Contains genes encoding plant cell wall-degrading enzymes, including pectin-degrading enzymes.
- Plays a role in the microbial degradation of lignocellulosic plant biomass .
Fungi
- Aspergillus spp.
- Aspergillus niger and Aspergillus aculeatus produce polygalacturonases.
- Hydrolyze α-1,4-glycosidic bonds in pectin, resulting in galacturonic acid.
- Widely used in industrial applications for pectin degradation .
- Penicillium spp.
- Penicillium notatum produces exo-polygalacturonase.
- Displays optimal activity at pH 6.0 and 50°C.
- Used in various applications requiring pectin degradation .
- Geotrichum reessii
- Yeast that produces protopectin-solubilizing enzymes.
- Degrades highly polymerized pectin and pectic acid oligomers.
- Found in soil and decaying vegetable matter .
Yeasts
- Saccharomyces cerevisiae
- Produces pectin methylesterase.
- Involved in the de-esterification of pectin, facilitating its degradation.
- Plays a role in the fermentation process by modifying pectin .
Enzymes Involved in Pectin Degradation
- Pectin Methylesterase (PME)
- Catalyzes the de-esterification of pectin into pectate and methanol.
- Found in plants, bacteria, and fungi.
- Plays a role in cell wall metabolism during fruit ripening and in microbial pathogenesis .
- Pectate Lyase (PL)
- Cleaves pectate through eliminative cleavage.
- Produces oligosaccharides with 4-deoxy-α-D-galact-4-enuronosyl groups.
- Involved in the maceration and soft rotting of plant tissue .
- Polygalacturonase (PG)
- Hydrolyzes α-1,4-glycosidic bonds in pectin.
- Produces galacturonic acid.
- Plays a role in fruit ripening and in the degradation of plant cell walls by pathogens .
Enzymes involved in the degradation of pectin – Pectinolytic enzymes
Pectin Methylesterase (PME) –
- EC Number: 3.1.1.11
- Function: De-esterifies pectin to yield pectate and methanol.
- Action: Modifies pectin’s methyl ester groups, facilitating further degradation.
- Significance: Plays a role in fruit ripening and pathogen-induced cell wall modification.
Pectin Lyase (PL) –
- EC Number: 4.2.2.10
- Function: Cleaves pectin via β-elimination, producing unsaturated oligosaccharides.
- Action: Endo-acting enzyme that breaks internal glycosidic bonds.
- Significance: Important in fruit juice clarification and textile retting processes. en.wikipedia.org+1en.wikipedia.org+1
Polygalacturonase (PG) –
- EC Number: 3.2.1.15
- Function: Hydrolyzes α-1,4-glycosidic bonds in pectin, releasing galacturonic acid.
- Action: Can be endo- or exo-acting; endo-PG cleaves randomly, exo-PG releases monomers from the non-reducing end.
- Significance: Key enzyme in fruit ripening and in the degradation of plant cell walls by pathogens.
Protopectinase –
- Function: Solubilizes protopectin, converting it into pectin.
- Action: Acts on highly methylated pectin forms, initiating their breakdown.
- Significance: Facilitates the initial step in pectin degradation, especially in plant tissues.
Rhamnogalacturonase (RGase) –
- Function: Degrades rhamnogalacturonan I, a pectin component.
- Action: Hydrolyzes α-1,4-glycosidic bonds between rhamnose and galacturonic acid.
- Significance: Contributes to the breakdown of complex pectin structures in plant cell walls.
Pectin Depolymerase –
- Function: Breaks down pectin into smaller oligosaccharides.
- Action: Hydrolyzes glycosidic bonds in pectin, reducing its molecular weight.
- Significance: Enhances the accessibility of pectin for further enzymatic degradation.
Polygalacturonase-Inhibiting Proteins (PGIPs) –
- Function: Inhibit polygalacturonase activity.
- Action: Bind to PG, preventing its interaction with pectin.
- Significance: Play a role in plant defense against pectin-degrading pathogens.
Pectinase (General Term) –
- Function: Refers to enzymes that degrade pectin.
- Action: Includes PME, PL, PG, and others.
- Significance: Widely used in industries like fruit juice extraction and textile processing.

Factors affecting pectin degradation
- Temperature – Enzyme activity escalates with temperature till reaching an optimal threshold, after which denaturation transpires. Pectinase enzymes typically demonstrate optimal activity at temperatures ranging from 45 to 55°C.
- pH – Each pectinase possesses an ideal pH range; for instance, pectinase enzymes often exhibit peak activity between pH 3.0 and 6.5.
- Enzyme Concentration – Elevated enzyme concentrations can expedite pectin degradation until reaching a saturation threshold where all substrate molecules are engaged.
- Substrate Concentration – Elevated substrate levels can augment breakdown rates until enzyme saturation occurs.
- Inhibitory Substances – Compounds such as heavy metals or certain compounds can impede pectinase activity, hence retarding breakdown.
- Co-factors – Certain pectinases necessitate metal ions such as Ca²⁺ or Mg²⁺ for maximal activity.
- Microbial Community – The diversity and composition of microbial communities can affect the rate and extent of pectin breakdown.
- Degree of Methylation -The degree of methylation indicates that pectin with a high degree of methylation exhibits greater resistance to degradation, whereas low-methylated pectin is more prone to destruction.
- Salt Concentration -Elevated salt concentrations can diminish enzyme function by interfering with ionic bonding.
- Oxygen Levels – Aerobic environments facilitate microbial proliferation and pectin decomposition, while anaerobic conditions may inhibit microbial activity.
Process of pectin degradation
Deesterification – The preliminary phase in pectin breakdown entails the elimination of methyl or acetyl groups from pectin molecules. This process is facilitated by enzymes including pectin methylesterases and pectin acetylesterases. Pectin methylesterases selectively hydrolyze the methyl ester groups of galacturonic acid units, yielding pectic acid and methanol. Pectin acetylesterases hydrolyze acetyl ester groups, yielding pectic acid and acetate. These esterases function before other enzymes such as polygalacturonases and pectate lyases, as they necessitate non-esterified substrates for optimal action.
Hydrolytic Cleavage – Subsequent to deesterification, the pectin backbone experiences hydrolytic cleavage of α-1,4-glycosidic bonds. This process is essential for deconstructing the pectin structure into smaller oligosaccharides. This process is facilitated by two principal types of enzymes:
- Polygalacturonases (PGs) – Polygalacturonases (PGs) are enzymes that hydrolyze the α-1,4-glycosidic linkages linking galacturonic acid residues within the pectin backbone. They can function as endo- or exo-enzymes, cleaving the pectin chain either randomly or from the termini, respectively. Cleavage yields either 6-methyl-D-galacturonate or D-galacturonate, contingent upon the enzyme type.
- Pectate Lyases (PLs)- These enzymes facilitate the eliminative cleavage of polygalacturonic acid, yielding unsaturated oligosaccharides via a trans-elimination process. This method contrasts with hydrolytic cleavage, as it yields unsaturated compounds.
Collectively, these enzymatic reactions result in the thorough breakdown of pectin, enabling its use by microbes in diverse ecological and industrial applications.
Mechanisms of microbial degradation of pectin
The following are some mechanism of action of enzymes involved in pectin degradation:
1. Mechanism of de-esterification by pectin methylesterases (PMEs)
- The single-chain mechanism where the enzyme acts sequentially along the polymeric chain removing methyl groups one after another
- The multiple-chain mechanism involving one catalytic event before releasing and rebinding to a new site
- A multiple-attack mechanism where PMEs catalyze several de-esterifications in one binding event
- Bacterial PMEs often use both single-chain and multiple-attack modes, producing adjacent de-esterified regions
- Fungal PMEs attack randomly via the multiple-chain mechanism, releasing protons that boost endo-polygalacturonase activity
- Example
- The de-esterification of galacturonan in Bacteroides species occurs predominantly by a multi-attack mechanism, followed by oligomer breakdown to monomers
2. Mechanism of hydrolytic cleavage by polygalacturonases (PGs)
- Active-site residues position on the α-1,4-glycosidic bond via multiple hydrogen bonds
- Hydrogen bonding induces strain and distortion on the glycosidic linkage
- Proton transfer between catalytic residues and the bond cleaves the glycosidic linkage, releasing the first end product and forming a covalent enzyme–substrate intermediate en.wikipedia.org
- A water molecule is then positioned for nucleophilic attack, releasing the second product and regenerating the active site
- Example
- In Rhizopus oryzae, about 18 endo- and exo-PGs plus one β-galactosidase cooperatively hydrolyze the α-1,4-linkages by this two-step mechanism
3. Mechanism of β-elimination by pectate lyases (PLs) and pectin lyases
- PLs bind de-esterified galacturonic acid regions and abstract a proton at C-5, facilitating β-elimination of the glycosidic bond
- This generates an unsaturated oligo-galacturonide with a 4,5-unsaturated non-reducing end
- No water is required for bond cleavage, making the reaction rapid under alkaline conditions
- Example
- Bacillus-derived PLs efficiently perform β-elimination on pectate, yielding unsaturated oligomers used in industrial processes
4. Mechanism of side-chain removal by rhamnogalacturonan hydrolases and lyases
- Rhamnogalacturonan hydrolases hydrolyze the α-1,2-linkage between rhamnose and galacturonic acid
- Rhamnogalacturonan lyases cleave via β-elimination, targeting the same linkages
- These enzymes unmask homogalacturonan regions for subsequent action by PGs and PLs
Microbial producers for pectinases
- Aspergillus niger – filamentous fungus widely used for industrial pectinase production under submerged fermentation.
- Penicillium occitanis – hyperproducing mutant Pol6 yields exceptionally high pectinase alongside cellulase.
- Rhizopus oryzae – secretes a suite of endo- and exo-polygalacturonases for efficient pectin hydrolysis.
- Rhizopus niveus – saprophytic filamentous fungus used industrially for pectinase enzyme production.
- Bacillus subtilis – soil bacterium isolated from fruit peels that produces extracellular pectinases.
- Erwinia chrysanthemi – plant-pathogenic bacterium renowned for secreting potent pectin-degrading enzymes.
- Pseudomonas fluorescens – Gram-negative bacterium with pectinolytic activity across broad pH ranges.
- Streptomyces lydicus – actinomycete producing pectinases under solid-state fermentation conditions.
- Yarrowia lipolytica – non-conventional yeast exhibiting pectinolytic enzymes useful in juice clarification.
- Saccharomyces cerevisiae – yeast strain engineered or naturally possessing low-level pectinase activity for mild fruit processing.
Applications of pectinase
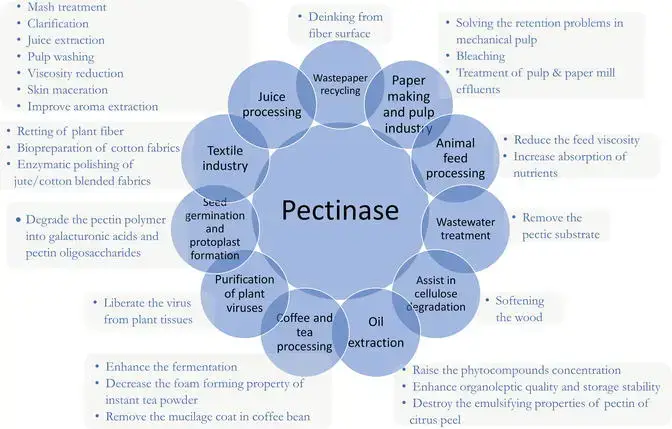
- Fruit juice clarification and extraction – speeds up liquefaction of fruit pulp and enhances yield in apple, grape, and citrus juice processing
- Wine and beer clarification – degrades pectin haze, intensifies color and aroma extraction during fermentation
- Tea and coffee fermentation – accelerates breakdown of cell‐wall pectins to improve flavor release and reduce steeping time.
- Textile retting and bioscouring – degums plant fibers (ramie, jute, flax) under mild conditions, replacing harsh chemicals.
- Paper and pulp industry – improves pulp bleachability, fines retention and paper smoothness by removing pectic substances.
- Wastewater treatment – degrades pectic residues in effluents from fruit‐processing plants, lowering COD and facilitating clean‐up.
- Animal feed enhancement – increases bioavailability of nutrients by breaking down plant cell‐wall pectins in feed formulations.
- Oil extraction from seeds and olives – aids release of vegetable oils by hydrolyzing cell‐wall components.
- Plant virus purification – assists in freeing virus particles from host tissues by degrading pectic barriers.
- Concrete crack healing and drug delivery research – employed in biotechnological applications to mediate polymer crosslinking and controlled release
FAQ
What is pectin?
Pectin is a naturally occurring polysaccharide found in many fruits and vegetables, particularly in the cell walls of ripe fruits.
What is the function of pectin in plants?
Pectin helps to give plants structure and support, by binding together the cell walls. It also plays a role in the ripening and softening of fruits.
What are pectinases?
Pectinases are enzymes that break down pectin molecules. They are produced by microorganisms, such as bacteria and fungi, as well as by some plants.
What are the industrial applications of pectinases?
Pectinases are widely used in the food industry to break down pectin in fruits and vegetables, and to improve the texture and clarity of juices and other beverages. They are also used in the production of textiles, paper, and biofuels.
What are the different types of pectinases?
There are several types of pectinases, including polygalacturonases, pectin lyases, and pectin methylesterases. Each type of enzyme targets a specific part of the pectin molecule.
- Abbott DW, Boraston AB. Structural biology of pectin degradation by Enterobacteriaceae. Microbiol Mol Biol Rev. 2008 Jun;72(2):301-16, table of contents. doi: 10.1128/MMBR.00038-07. PMID: 18535148; PMCID: PMC2415742.
- Mosaad Khattab, A. (2022). The Microbial Degradation for Pectin. Pectins – The New-Old Polysaccharides. doi: 10.5772/intechopen.100247
- Haile, S., Masi, C. & Tafesse, M. Isolation and characterization of pectinase-producing bacteria (Serratia marcescens) from avocado peel waste for juice clarification. BMC Microbiol 22, 145 (2022). https://doi.org/10.1186/s12866-022-02536-8
- Abbott DW, Boraston AB. Structural biology of pectin degradation by Enterobacteriaceae. Microbiol Mol Biol Rev. 2008 Jun;72(2):301-16, table of contents. doi: 10.1128/MMBR.00038-07. PMID: 18535148; PMCID: PMC2415742.
- Manucharova, Natalia. (2009). The microbial destruction of chitin, pectin, and cellulose in soils. Eurasian Soil Sci. 42. 1526-1532. 10.1134/S1064229309130146.
- Satapathy, S., Rout, J. R., Kerry, R. G., Thatoi, H., & Sahoo, S. L. (2020). Biochemical Prospects of Various Microbial Pectinase and Pectin: An Approachable Concept in Pharmaceutical
- Bioprocessing. Frontiers in Nutrition, 7. doi:10.3389/fnut.2020.00117
Wang B, Sun Z, Yu Z. Pectin Degradation is an Important Determinant for Alfalfa Silage - Fermentation through the Rescheduling of the Bacterial Community. Microorganisms. 2020; 8(4):488. https://doi.org/10.3390/microorganisms8040488
- https://www.ars.usda.gov/ARSUserFiles/60701000/Pickle%20Pubs/p294.pdf
- https://core.ac.uk/download/pdf/30268675.pdf
- https://www.cabdirect.org/cabdirect/abstract/19931378691
- https://www.jstor.org/stable/43237134
- https://worldwidescience.org/topicpages/p/pectin+degradation+pathway.html
- https://openbiotechnologyjournal.com/contents/volumes/V3/TOBIOTJ-3-9/TOBIOTJ-3-9.pdf
- https://d-nb.info/111260894X/34
- https://www.studocu.com/en-gb/document/university-of-salford/biology/lecture-notes-the
- microbial-degradation-of-pectin/16116366
- https://www.sciencedirect.com/science/article/abs/pii/S0141813023047037
- https://ift.onlinelibrary.wiley.com/doi/10.1111/1750-3841.16233