What is lignin?
Lignin is a complex, high-molecular-weight polymer made mostly of phenolic compounds. It is found in the cell walls of vascular plants, especially in the secondary cell wall during the development of xylem, phloem fibres, and sclerenchyma cells, which give the plant strength, support, and waterproofing.
It is made up of three main monolignols: coniferyl alcohol, sinapyl alcohol, and p-coumaryl alcohol. These monolignols are made through the phenylpropanoid pathway, which starts with the amino acid phenylalanine, and then they are polymerised in the cell wall matrix by oxidative enzymes like peroxidases and laccases.
This is why lignin makes the cell walls stronger: it embeds itself in and cross-links with cellulose and hemicellulose fibres, which makes the plant tissues stiff and able to handle mechanical stress. It also makes the plant tissues water-repellent, which keeps water from escaping and lets water flow easily through the vascular tissues.
Lignin also protects plants from diseases by creating physical barriers that make it hard for microbes to invade and spread through the plant’s tissues.
Lignin is hard to break down because it has a complicated and irregular polymer structure. Most organisms can’t break it down easily, but some fungi and bacteria make enzymes like lignin peroxidase, manganese peroxidase, and laccase that help break down lignin during decomposition.
In the paper and biofuel sectors, lignin is frequently seen as a waste. However, because it is abundant and renewable, researchers are looking at how it could be used to make bio-based materials, chemicals, and renewable energy sources.
Structure of lignin
Lignin Structure –
- Phenylpropane Units – Lignin is a complex polymer composed of phenylpropane units, which are interconnected through various chemical linkages, including alkyl-alkyl, alkyl-aryl, and aryl-aryl groups.
- Monolignol Precursors – The primary precursors for lignin synthesis are p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol.
- Functional Groups – Lignin contains several functional groups such as alcohol hydroxyl, carbonyl, carboxyl, phenolic hydroxyl, methoxyl, and sulfonic acid groups.
- Elemental Composition – Lignin is primarily composed of carbon, hydrogen, and oxygen, with a higher carbon content compared to homogeneous carbohydrates.
- Structural Complexity – The molecular structure of lignin is highly irregular and cannot be described by a simple structural formula, making its chemical structure one of the most challenging problems in the field of natural polymers.
Linkages Between Units –
- Types of Linkages – Lignin’s phenylpropane units are connected through various linkages, including ether bonds (such as β-O-4, α-O-4, 4-O-5) and carbon-carbon bonds (such as β–β, β-5, 5–5).
- Prevalence of β-O-4 Linkage – The β-O-4 ether linkage is the most abundant inter-unit linkage in lignin, occurring at levels of 40–60% in softwood and hardwood.
- Species Variation – The relative abundance of each linkage varies among different plant species, affecting the overall structure and properties of lignin.
Biosynthesis and Tissue Distribution –
- Biosynthetic Pathway – Lignin is biosynthesized through the dehydrogenative polymerization of monolignols, which are synthesized via the phenylpropanoid pathway from the amino acids phenylalanine or tyrosine.
- Tissue Variation – Lignin content and structure can vary significantly across different plant tissues and species, influenced by ecological factors such as plant growth, nutrition, climate, and illumination.
Chemical Structure Challenges –
- Irregular Coupling – The formation of lignin involves the irregular coupling or addition of monolignol precursors, leading to a complex and heterogeneous structure.
- Lack of Optical Activity – Despite the presence of many asymmetric centers in lignin molecules and their degradation products, lignin does not exhibit optical activity, distinguishing it from polymers like cellulose or proteins.
- Analytical Challenges – The complex structure of lignin makes it difficult to determine its molecular structure through general decomposition methods, necessitating specialized analytical techniques.
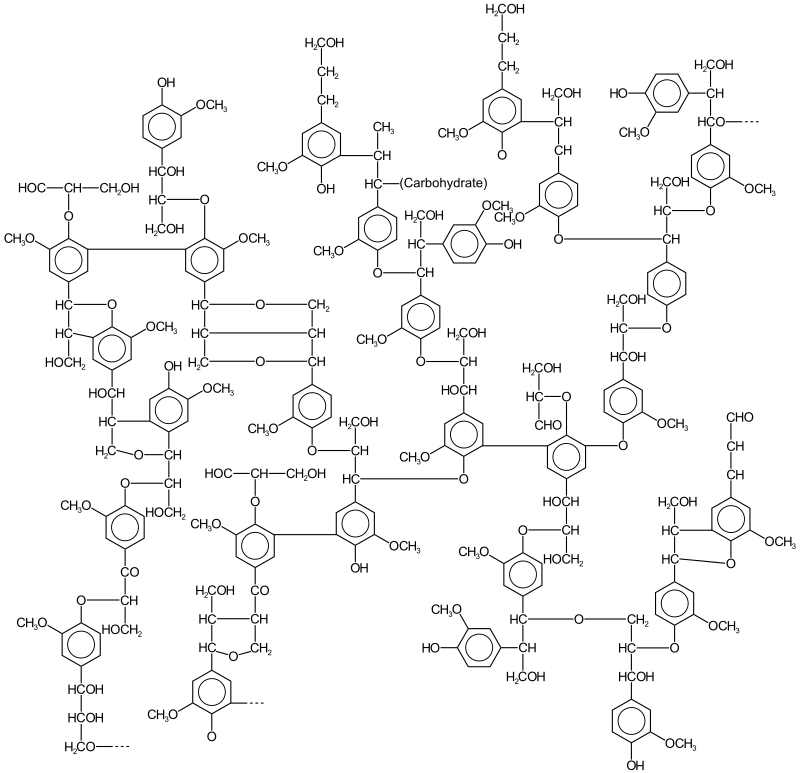
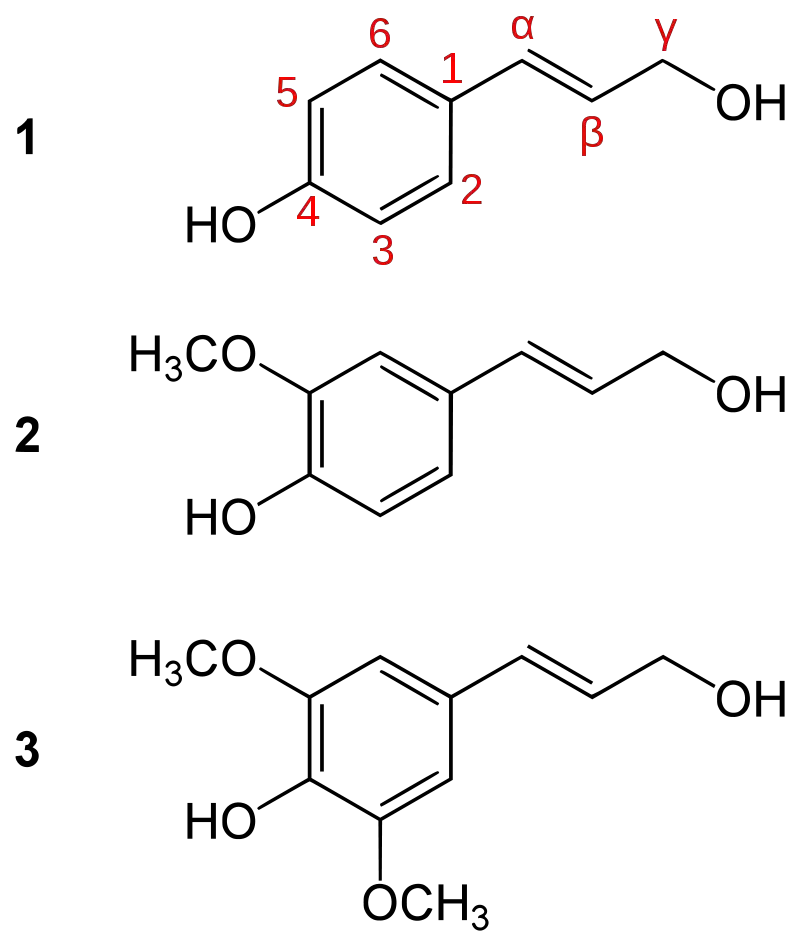
Biological function of lignin
- Structural Support and Rigidity- Lignin fills the crevices between cellulose and hemicellulose in cell walls. This gives plant tissues like xylem and sclerenchyma the mechanical strength, resistance to compression, and stiffness they need to grow out of water and form a forest canopy.
- Water Transport Facilitation- Lignin is hydrophobic, which means it keeps water from leaking out of xylem walls. This lets water move more easily up through vascular tissues.
- Barrier against Pathogen Invasion- When a plant is hurt or attacked by a pathogen, lignin builds up around the site of the threat, physically stopping fungal enzymes, poisons, and pests from getting in and spreading.
- Protection Against Stress
- from abiotic challenges such drought, UV radiation, high temperatures, and heavy metals. Under stress, lignin deposition increases to stabilise cell walls.
- against biotic stressors, which it protects against by functioning as both a physical barrier and a chemical defence, often through oxidative processes. Its biosynthetic genes are turned on when insects and pathogens attack it.
- Development and Lodging Resistance- Lignification makes stems, roots, and seed coats stronger during development. This helps crops like wheat avoid lodging by making cell walls thicker and making the structure more stable.
- Seed Protection and Dispersion– Lignin makes seed coats and other cells strong and sometimes controls dehiscence, which helps with dispersal mechanisms.
- Evolutionary Innovation – The appearance of lignin in vascular plants about 430 million years ago was a key milestone in evolution since it was necessary for upright growth and water transport, which made it possible for plants to live on land.
Discovery of novel ether‑cleaving enzymes – new microbes capable of cleaving ether bonds in lignin model compounds (like 2‑PAP) via previously unknown enzymes have been isolated, expanding the toolkit for industrial lignin valorization
Factors affecting lignin degradation
- Microbial Factors –
- Microbial Community Composition – The diversity and abundance of microorganisms, including bacteria and fungi, significantly influence lignin degradation. White-rot fungi such as Phanerochaete chrysosporium and Trametes versicolor are particularly effective due to their production of ligninolytic enzymes.
- Enzyme Production – Lignin degradation is facilitated by enzymes like lignin peroxidases (LiP), manganese peroxidases (MnP), versatile peroxidases (VP), dye-decolorizing peroxidases (DyP), and laccases. The production and activity of these enzymes are regulated by environmental factors and nutrient availability.
- Oxygen Availability – Oxygen is essential for the activity of certain ligninolytic enzymes. Aerobic conditions typically enhance the efficiency of lignin degradation, whereas anaerobic conditions can limit enzymatic activity.
- Environmental Factors –
- Temperature – The activity of ligninolytic enzymes is temperature-dependent. Optimal temperatures vary among species, but extreme temperatures can denature enzymes and inhibit lignin degradation.
- pH Levels – The pH of the environment affects enzyme activity and microbial growth. Acidic conditions often enhance the activity of ligninolytic enzymes, whereas alkaline conditions may reduce their effectiveness.
- Moisture Content – Adequate moisture is necessary for microbial growth and enzyme activity. Low moisture levels can lead to desiccation of microorganisms, reducing lignin degradation rates.
- Substrate Characteristics –
- Lignin Concentration – High concentrations of lignin can be inhibitory to microbial growth and enzyme activity. Dilution or pretreatment of lignin can enhance degradation efficiency.
- Lignin Structure – The chemical structure of lignin, including the presence of different monolignols and the types of linkages, affects its susceptibility to degradation. For instance, syringyl lignin is more readily degraded than guaiacyl lignin due to its lower redox potential.
- Ecological Factors –
- Nutrient Availability – The presence of other nutrients, such as nitrogen and sulfur, can influence enzyme production and microbial growth, thereby affecting lignin degradation.
- Plant Tissue Type – Different plant tissues, such as leaves, stems, and roots, have varying lignin contents and structures, which can impact the rate of lignin degradation.
- Anthropogenic Factors –
- Pollution – Environmental pollutants can inhibit microbial activity and enzyme function, thereby reducing lignin degradation rates.
- Land Use Practices – Agricultural and forestry practices can alter environmental conditions and microbial communities, influencing lignin degradation processes.
Lignin-degradating Microorganisms
White-rot fungi –
- Phanerochaete chrysosporium – A model organism for lignin degradation, producing lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase (Lac) enzymes.
- Trametes versicolor – Known for its ability to degrade various lignin components and environmental pollutants.
- Pleurotus ostreatus – Efficient in breaking down lignin and cellulose, contributing to wood decay.
- Ceriporiopsis subvermispora – Selective in lignin degradation, leaving cellulose intact for further processing.
- Cyathus stercoreus – Produces laccase and MnP, effectively decomposing lignin in agricultural residues.
Brown-rot fungi –
- Serpula lacrymans – Causes dry rot in timber by degrading cellulose and hemicellulose, leaving lignin largely intact.
- Fomitopsis pinicola – Attacks lignin through non-enzymatic oxidation, producing hydroxyl radicals via Fenton chemistry.
- Phaeolus schweinitzii – Decomposes lignin in wood, contributing to forest ecosystem nutrient cycling.
Soft-rot fungi –
- Chaetomium – Degrades lignin in decaying wood, especially under moist conditions.
- Ceratocystis – Attacks lignin in wood, often associated with plant diseases.
- Kretzschmaria deusta – Decomposes lignin in wood, contributing to forest nutrient cycling.
Ligninolytic bacteria –
- Streptomyces viridosporus – Produces lignin peroxidase, effective in degrading lignin and humic acids.
- Streptomyces badius – Degrades Kraft lignin, producing extracellular ligninases.
- Bacillus aryabhattai BY5 – Demonstrates lignin degradation capabilities in alkaline conditions.
- Acinetobacter johnsonii LN2 – Degrades alkaline-lignin, producing ligninolytic enzymes.
- Micrococcus yunnanensis CL32 – Exhibits high manganese peroxidase activity, aiding in lignin degradation.
- Burkholderia sp. H1 – Degrades lignin under limited oxygen conditions.
- Klebsiella sp. BRL6-2 – Lignin-degrading bacterium isolated from tropical forest soils.
- Mangrovibacterium lignilyticum – Facultatively anaerobic bacterium isolated from mangrove sediments, capable of lignin degradation.
Enzymes involved in lignin degradation –
- Laccase (Lac) – Oxidizes phenolic compounds in lignin, producing free radicals that break lignin bonds.
- Lignin peroxidase (LiP) – Catalyzes the oxidative cleavage of lignin structures.
- Manganese peroxidase (MnP) – Oxidizes Mn²⁺ to Mn³⁺, which then oxidizes lignin.
- Versatile peroxidase (VP) – Combines features of LiP and MnP, capable of degrading a wide range of lignin structures.
- Arylhydrocarbon dioxygenase (AhD) – Involved in the oxidative cleavage of lignin components.
Lignin-degradating Enzymes
- Lignin peroxidase (LiP) – a heme‑containing enzyme that, using hydrogen peroxide as an oxidant, can catalyze direct oxidative cleavage of non‑phenolic lignin structures via long‑range electron transfer, enabling degradation of complex aromatic units.
- Manganese peroxidase (MnP) – a heme peroxidase that oxidizes Mn²⁺ to Mn³⁺, and the resulting Mn³⁺ diffuses to and oxidizes phenolic lignin units; produced by many white‑rot fungi and some bacteria, optimally active between pH 3.5–9 and 25–70 °C.
- Versatile peroxidase (VP) – a multifunctional heme peroxidase that has catalytic features of both LiP and MnP, capable of oxidizing a broad spectrum of lignin substrates.
- Laccase – a multicopper oxidase that oxidizes phenolic and some non‑phenolic lignin moieties by four‑electron reduction of O₂ to H₂O, found in fungi and bacteria (e.g., Pseudomonas, Bacillus), often acting synergistically with peroxidases.
- Dye‑decolorizing peroxidase (DyP‑type) – bacterial heme peroxidases identified in Rhodococcus, Amycolatopsis, etc., capable of oxidizing lignin related compounds and synthetic dyes, often working intracellularly or extracellularly.
- Auxiliary enzymes (LDA) – non‑LME enzymes such as aryl‑alcohol oxidases, glyoxal oxidases, and oxalate oxidases that generate H₂O₂ or reactive radicals essential for the activity of peroxidases.
- Heme‑thiolate peroxidases (HTP) – recently discovered in Ceriporiopsis subvermispora, these enzymes share catalytic traits with P450s and catalases and oxidize aryl alcohols, suggesting potential roles in lignin breakdown
Lignin degradation Process
Here’s a simple sequence, in straightforward steps, of microbial lignin degradation:
- Step 1 – Depolymerization – extracellular lignin‑modifying enzymes (LiP, MnP, VP, DyP, laccase) oxidatively fragment complex lignin into smaller phenolic/non‑phenolic compounds
- Step 2 – Solubilization – fragmented lignin becomes water‑soluble low‑molecular‑weight aromatic molecules that can diffuse or be transported into cells
- Step 3 – Uptake – microbes take up the soluble aromatic fragments via transport systems, especially bacteria
- Step 4 – Intracellular catabolism – aromatic fragments are metabolized via pathways like β‑ketoadipate or gentisate, feeding into the TCA cycle as intermediates
- Step 5 – Mineralization – further metabolism converts compounds into CO₂, H₂O, and microbial biomass
That’s it—five clear steps: fragmentation outside the cell, solubilization, uptake, internal breakdown, and full mineralization.
Microbial degradation Mechanisms of lignin
- Step 1 – In vitro oxidative depolymerization by extracellular enzymes
- Laccase activity – catalyzes oxidation of phenolic units first, then, via mediators, initiates oxidation of non-phenolic benzylic structures; proceeds via electron transfer, radical H-atom abstraction, and ionic pathways, leading to Cα–Cβ cleavage and solubilization of lignin fragments as lignin–mediator complexes
- Lignin peroxidase (LiP) & Manganese peroxidase (MnP) – initiate one-electron oxidation using H₂O₂, generate Mn³⁺ (in MnP) which oxidizes phenolic substrates; radicals produced then attack lignin, cleave arylglycerol-aryl ether bonds, and produce smaller aromatic products like ketones
- Step 2 – Solubilization of lignin fragments
- Formation of smaller hydrophilic molecules (e.g., phenolics, aldehydes, ketones) due to enzymatic cleavage; these can now be absorbed into microbial cells for further intracellular processing
- Step 3 – In vivo intracellular degradation by catabolic pathways
- β-O-4 ether cleavage (main lignin linkage)
- LigD oxidizes Cα-OH, LigE/LigF cleave β-O-4 bond → generates vanillin & GS-HPV
- LigG removes glutathione from GS-HPV → oxidized to vanillin
- Biphenyl bond cleavage (minor linkage)
- LigX demethylates DDVA → LigZ cleaves via meta-cleavage → LigY hydrolyzes it → yields 5-carboxyvanillic acid (5CVA) and 4-carboxy-2-hydroxypentadienoic acid
- β-O-4 ether cleavage (main lignin linkage)
- Step 4 – Ring fission and mineralization
- Compounds like vanillin or protocatechuate are subjected to ring-cleaving dioxygenases (e.g., protocatechuate 4,5-dioxygenase in Pseudomonas)
- Generates TCA cycle intermediates, CO₂, and water → complete microbial assimilation of lignin carbon
- Step 5 – Example pathway in Pseudomonas
- Benzaldehyde lyase cleaves acyloin linkages in lignin-derived diarylethenes
- Protocatechuate ring-cleaved into central metabolic intermediates via 4,5-dioxygenase
FAQ
What is lignin and why is it important?
Lignin is a complex polymer that provides structural support to plant cell walls. It is the second most abundant organic material on earth after cellulose. Lignin is important because it contributes to the strength and rigidity of plants and is also a potential source of renewable energy and chemicals.
How do microorganisms degrade lignin?
Microorganisms degrade lignin by using ligninolytic enzymes such as peroxidases and laccases to cleave the lignin polymer into smaller subunits. They can also use other enzymes such as esterases and hydrolases to break the ester and ether bonds in lignin.
What factors affect microbial degradation of lignin?
Factors that can affect microbial degradation of lignin include pH, temperature, moisture content, nutrient availability, oxygen availability, and the presence of inhibitors such as heavy metals or pesticides.
What are some examples of microorganisms that can degrade lignin?
Microorganisms that are known to degrade lignin include bacteria, fungi, and actinomycetes. Examples of lignin-degrading microorganisms include Streptomyces viridosporus, Phanerochaete chrysosporium, and Bacillus subtilis.
How can lignin be valorized using microbial degradation?
Microbial degradation of lignin can be used to produce high-value products such as biofuels, bioplastics, and chemicals. By breaking down lignin into smaller subunits, microorganisms can generate feedstocks that can be used for the production of these products.
What are ligninolytic enzymes and how do they work?
Ligninolytic enzymes are a class of enzymes that are capable of breaking down lignin. They work by generating free radical intermediates that can cleave the lignin polymer into smaller subunits.
What are the challenges associated with microbial degradation of lignin?
One of the main challenges associated with microbial degradation of lignin is the complexity of the lignin polymer. Lignin is a highly heterogeneous material that is resistant to degradation. Another challenge is the variability in lignin composition, which can affect the ability of microorganisms to degrade lignin.
How can biotechnology be used to improve microbial degradation of lignin?
Biotechnology can be used to engineer microorganisms that are more efficient at degrading lignin. This can involve the optimization of ligninolytic enzyme production, the enhancement of lignin transport across cell membranes, and the development of synthetic microbial communities that are specialized for lignin degradation.
What are some potential applications of lignin-derived products?
Lignin-derived products can be used in a variety of applications, including as fuel additives, polymer feedstocks, and surfactants. They can also be used in the production of specialty chemicals and materials such as adhesives and carbon fiber.
How does microbial degradation of lignin contribute to the global carbon cycle?
Microbial degradation of lignin is an important process in the global carbon cycle. By breaking down lignin into smaller subunits, microorganisms release carbon back into the ecosystem where it can be used by other organisms. This contributes to the cycling of carbon through terrestrial ecosystems and the atmosphere.
- Crawford, D. L., & Crawford, R. L. (1980). Microbial degradation of lignin. Enzyme and Microbial Technology, 2(1), 11–22. doi:10.1016/0141-0229(80)90003-4
- Janusz, G., Pawlik, A., Sulej, J., Świderska-Burek, U., Jarosz-Wilkołazka, A., & Paszczyński, A. (2017). Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiology Reviews, 41(6), 941–962. doi:10.1093/femsre/fux049
- Reid, I. D. (1995). Biodegradation of lignin. Canadian Journal of Botany, 73(S1), 1011–1018. doi:10.1139/b95-351
- Geib, S. M., Filley, T. R., Hatcher, P. G., Hoover, K., Carlson, J. E., Jimenez-Gasco, M. d. M., … Tien, M. (2008). Lignin degradation in wood-feeding insects. Proceedings of the National Academy of Sciences, 105(35), 12932–12937. doi:10.1073/pnas.0805257105
- Bugg, T. D. H., Ahmad, M., Hardiman, E. M., & Rahmanpour, R. (2011). Pathways for degradation of lignin in bacteria and fungi. Natural Product Reports, 28(12), 1883. doi:10.1039/c1np00042j
- Atiwesh G, Parrish CC, Banoub J, Le TT. Lignin degradation by microorganisms: A review. Biotechnol Prog. 2022 Mar;38(2):e3226. doi: 10.1002/btpr.3226. Epub 2021 Dec 9. PMID: 34854261.
- Atiwesh, Ghada & Parrish, C. & Banoub, Joseph & Le, Tuyet-Anh. (2022). Lignin Degradation by Microorganisms: A Review. Biotechnology Progress. 38. 12. 10.1002/btpr.3226.
de Souza, W. R. (2013). Microbial Degradation of Lignocellulosic Biomass. InTech. doi: 10.5772/54325 - https://www.microbiology.ubc.ca/research/labs/eltis/research/bacterial-lignin-degradation
- https://www.frontiersin.org/articles/10.3389/fbioe.2019.00209/full
- https://core.ac.uk/download/pdf/78375034.pdf
- https://warwick.ac.uk/fac/sci/chemistry/research/bugg/bugggroup/research/lignin/
Well written