Membrane Filter Technique can define as a physical separation process, which based mainly on size / molecular weight of particles in a liquid or gas stream.
In this method a thin semi-permeable membrane (often made from cellulose nitrate/acetate) is used, and pressure is applied to push the feed solution by it.
The smaller components pass through as permeate, while larger solids or microbes are retained as retentate.
This process sometimes termed as Millipore filter technique or membrane separation—depending by application.
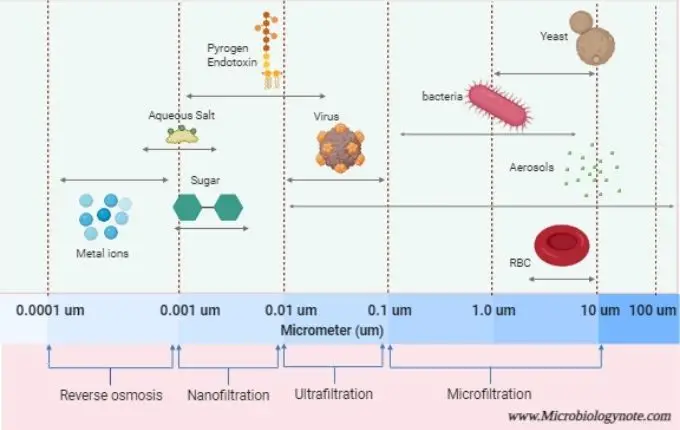
The main types are Microfiltration (MF), Ultrafiltration (UF), Nanofiltration (NF) and Reverse Osmosis (RO); arranged in increasing order of separation fineness.
MF/UF are low-pressure system used for removing suspended solids and microbial cells; NF/RO works under higher pressure to remove ions, dissolved salts and small organic molecules.
Historically, it was first used around late 1950s for microbiological testing of water after study published in 1951.
The cellulose nitrate membrane filters were found effective to capture bacteria from water samples—so this technique became standard for monitoring potable water quality.
It’s widely applied in water and wastewater treatment, food & beverage, and pharmaceuticals industries for producing high-quality fluids.
The system also has advantage of small footprint, less energy need, and low chemical input compared with traditional treatment system, which makes it sturdy and hardy choice for modern facilities.
Principle of Membrane filtration/Membrane filtration mechanism
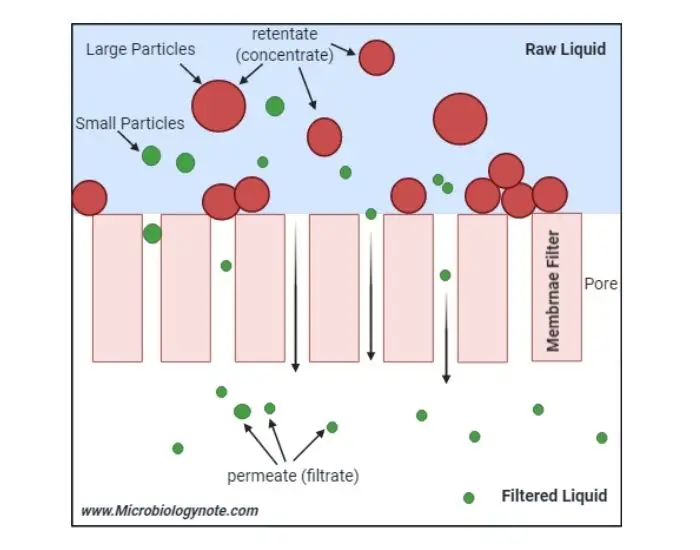
The principle of Membrane Filtration mainly based on size exclusion or straining mechanism which separates solutes / particles by their size or molecular weight.
A membrane act as a thin selective barrier that allows smaller molecules to pass through while larger ones are retained on the surface.
Usually, the driving force is pressure difference across the membrane; sometimes also concentration gradient or electric potential used for same purpose.
When feed solution pushed by membrane, only particles smaller than pore size (like 0.45 μm in case of bacterial test) move into permeate (filtrate), and others remain as retentate.
In microbiological analysis, the membrane works like two-dimensional screen where microorganisms larger than pores get trapped directly upon filter surface for later counting.
In Microfiltration (MF) and Ultrafiltration (UF), the removal depends mostly on physical straining, while Nanofiltration (NF) and Reverse Osmosis (RO) rely on diffusive mechanism linked with charge and size property.
Thus, separation process controlled by several factors — pressure, solute concentration, and molecular interactions inside the thin membrane matrix.
It can say that membrane act both as physical sieve and selective medium, giving sturdy and consistent separation performance.
Types of membrane filtration
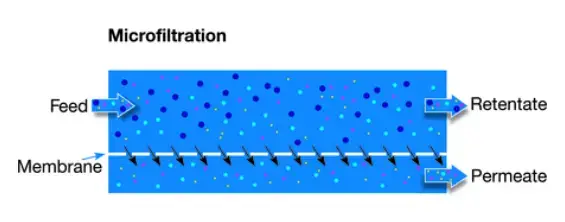
1. Microfiltration (MF)–
- MF is classified as low-pressure system, typically operated at about 10–30 psi, and pore sizes are given as 1.0–0.01 μm (sometimes noted 0.1–10 μm).
- Large particles, suspended solids, algae, clay, and bacteria are retained by the membrane, while water and small solutes pass through.
- For microbiological test a 0.45 μm filter is commonly used to trap bacteria on the surface, for later counting.
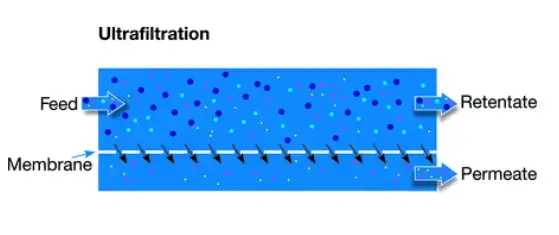
2. Ultrafiltration (UF) –
- UF is also low-pressure (≈ 10–30 psi) and pores are smaller, around 0.01–0.001 μm (10–100 nm), so macromolecules are retained.
- Proteins, viruses, colloids, pyrogens and starches are removed by straining and some adsorption occurs on the membrane surface.
- It is used for solid-liquid separation in water treatment and pharma, and it’s valued for producing sterile-like water.
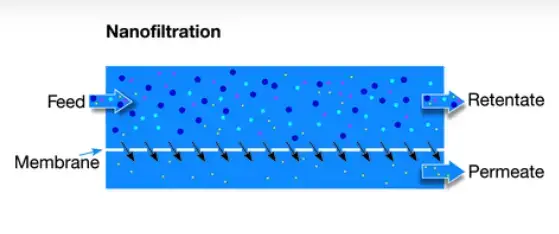
3. Nanofiltration (NF)–
- NF is a higher-pressure process (roughly 75–250 psi) and pore size ~ 0.001–0.0001 μm (1–10 nm).
- Multivalent ions (Ca²⁺, Mg²⁺), dyes, sugars, pesticides, herbicides and divalent anions are largely retained while monovalent ions pass more readily.
- Separation is influenced by both size exclusion and charge-interaction, so selectivity is partially electrostatic.
4. Reverse Osmosis (RO)–
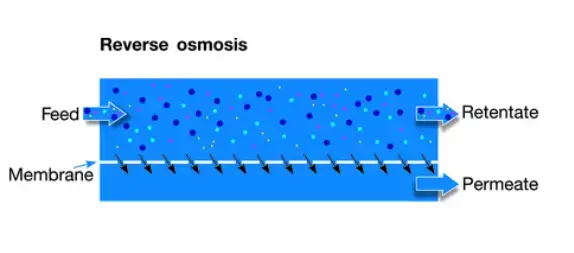
- RO is the tightest membrane process, pressure usually 75–250 psi, membrane described as dense/non-porous with pore size < ~1 nm (≈0.0001 μm).
- Nearly all dissolved salts, monovalent ions, and very small organic molecules are rejected, only H₂O molecules are allowed through.
- The process is highly effective, but it is energy demanding, and pre-treatment is often required to prevent fouling.
5. Electrodialysis (ED) / RED–
- ED (and RED) is electrically driven, ions are moved through ion-exchange membranes under voltage, and water flow is not the driving force.
- It is used for desalination and ion separation, when selective ion removal is needed, rather than particulate removal.
6. Forward Osmosis (FO)–
- FO operates by osmotic gradient, not by applied hydraulic pressure, and membranes similar to RO are commonly used.
- Solutes move toward the draw solution; water is drawn across membrane, it’s useful for water recovery and lower-energy desalting, though reconcentration of draw is required.
7. Membrane Distillation (MD) –
- MD is thermally driven, where vapor (not liquid) passes through a microporous hydrophobic membrane using a temperature difference.
- It is applied for desalination and for producing very pure water (vapor-condensed) and works at low pressure but needs heat input.
8. Gas Separation/ Permeation–
- Gas mixtures are separated by differences in permeability/permeation rates through polymeric or inorganic membranes; selectivity depends on size, diffusivity and solubility.
- Applications include O₂/N₂ separation, CO₂ removal, and specialty gas purification in industry.
Procedure of Membrane Filtration
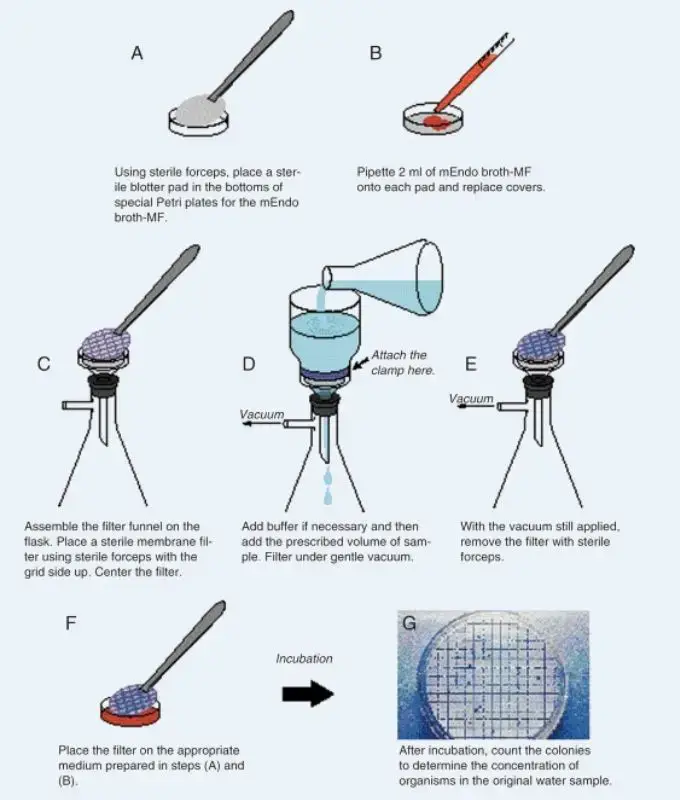
- Samples should be collected in sterile containers, and the volume is decided by expected microbial load (for drinking water commonly 100 mL).
- The sample is mixed gently by inverting several times, then an aliquot or calculated dilution is measured for filtration to aim for ~20–200 CFU per filter.
- The nutrient medium is prepared and Petri dishes are labeled on bottom with sample ID, date and volume, and if broth is used a sterile absorbent pad is placed and saturated (2–3 mL) then excess poured off.
- The filtration apparatus (Buchner funnel / filtration unit) is autoclaved earlier, and forceps are sterilized by ethanol dip then passed through flame, care being taken not to overheat.
- A sterile membrane filter (47 mm, 0.45 μm, grid-marked) is aseptically placed on the porous plate, grid side up, using sterile forceps, and the funnel is attached without disturbing the filter.
- About 30 mL sterile dilution water is poured into the funnel to wet the membrane, a brief vacuum is applied to confirm wetting and seal (if sample <10 mL then 10–20 mL sterile water used first).
- The measured sample volume is poured into funnel and vacuum (line, electric or hand pump) is applied so sample is drawn completely through, the membrane trapping microorganisms larger than pore-size.
- The funnel walls are rinsed twice with ≈30 mL sterile dilution water and vacuum applied again to pass rinse through the membrane, ensuring transfer of organisms to the filter surface.
- The funnel is removed carefully, and the membrane is lifted at the edge with sterile forceps, grid side up, then transferred onto the prepared agar or saturated pad using a rolling action to avoid air-bubbles and ensure full contact.
- Plates are incubated at appropriate temperature (e.g., 35 °C ±0.5 °C) for the required time (commonly ~24 h), and colonies are allowed to develop on the membrane surface.
- After incubation colonies are inspected (stereo microscope 10–15× or magnifier); on MI agar blue colonies indicate E. coli and fluorescence under UV (366 nm) is checked for total coliforms.
- Colonies are counted and results calculated as CFU per 100 mL, and suspicious colonies may be subcultured/stained for further biochemical confirmation.
- Controls are run to check sterility of membrane filters, dilution water and media (no-sample blanks), and equipment sterility is maintained to prevail contamination errors.
Factors that affect the performance of membrane filtration
There are several factors that can affect the performance of membrane filtration, including:
- Pore size of membrane – if pores are too large, microorganisms may pass through; too small can cause clogging & slow flow.
- Membrane material affects chemical resistance and compatibility with sample (like cellulose nitrate, polycarbonate, etc.).
- The sample turbidity and presence of suspended solids can block membrane pores quickly, reducing filtration rate.
- Pressure/vacuum applied during process influences the rate of flow and total recovery of microorganisms.
- The temperature of filtration system sometimes alters membrane flexibility and microbial survival.
- pH and ionic strength of sample solution can influence the attachment of microorganisms on filter surface.
- The type of organism present (size, shape, motility) also affects retention efficiency.
- Volume of sample filtered — larger volume causes more stress on membrane and may lead to rupture or clogging.
- Sterility and handling technique of operator plays crucial role, contamination may occur by poor aseptic practice.
- The storage condition of membrane (humidity, light exposure, etc.) can degrade filter quality over time.
- Sometimes the medium used after filtration also decides recovery rate, as not all bacteria grow on same nutrient.
- Flow rate adjustment is important – too fast may cause breakage, too slow makes process inefficient.
Advantages of Membrane Filtration
- The MF technique allows direct enumeration of microorganisms from large volumes of liquid, which gives more reliable data.
- It’s highly accurate and sensitive because even low microbial count can be detected easily.
- The method provides quantitative results, colonies developed on the membrane can be counted directly.
- As no heat is used, it’s suitable for heat-sensitive materials like vitamins, proteins, and enzymes.
- The filtration process is clean and requires less preparation of sample compared to pour plate or spread plate method.
- It allows rapid detection, as filters can be incubated immediately after sample collection.
- The membrane can be used for selective detection, using differential or selective media for specific bacteria like E. coli.
- Very small quantities of sample are lost during testing, thus making it ideal for expensive or limited materials.
- The method is portable, field-friendly, and useful in places without large lab setups.
- It gives clear and isolated colonies, so further biochemical identification becomes easy and faster.
Disadvantages of Membrane Filtration
- The membrane filters are quite fragile and can tear or clog easily, especially when used with turbid or viscous samples.
- It requires high maintenance, as membrane must be handled carefully and changed frequently.
- The process is time consuming when large volumes of liquid are filtered, clogging slows the rate drastically.
- Some microorganisms may get trapped inside filter pores or die during filtration, causing inaccurate results sometimes.
- It’s not suitable for samples containing high solids, since particles block the membrane quickly.
- The cost of membrane units and vacuum systems are relatively high, so operation becomes expensive for routine testing.
- Certain bacteria (like Streptococcus or Legionella) do not recover well on filter surface, so detection efficiency is reduced.
- There is a risk of contamination during handling or transfer of the membrane to culture medium.
- Some chemicals in the sample can damage or react with filter material, changing its efficiency/permeability.
- The method needs trained personnel because improper technique can lead to loss of sample or false-negative results.
Application of Membrane Filtration
- It is widely used for microbiological analysis of water, milk, and other beverages, mainly to detect coliforms, E. coli, etc.
- The technique is used to examine contamination in pharmaceutical & cosmetic products, especially for sterility testing.
- It can be applied in quality control of treated water (like in sewage treatment plants) for monitoring bacterial load.
- MF method is also employed for air monitoring, where airborne microorganisms are trapped on membrane for further culture.
- Used in food industry to check microbial safety of liquids – juices, soft drinks, and wine samples are usually tested.
- It’s also used in antibiotic assay, where the inhibition zones formed by bacterial growth around membrane are observed.
- Membrane filtration is used in sterilization process of heat-sensitive solutions, like vitamin, enzyme, or serum.
- In research laboratories, it’s used to separate or concentrate microbial cells, spores or particulate matter for study.
- This technique also helps in bacteriological analysis of swimming pool & recreational water (for health monitoring purpose).
- Sometimes it used for wastewater analysis, determining microbial population after various treatment stages.
Most Probable Number (MPN) VS Membrane Filtration Technique
| Most Probable Number (MPN) technique | Membrane filter technique |
| Slower: requires 48 hours for a positive | More rapid: quantitative results in or presumptive positive about 18 hours |
| More labor-intensive | Less labor-intensive |
| Requires more culture medium | Requires less culture medium |
| Requires more glassware | Requires less glassware |
| More sensitive | Less sensitive |
| Result obtained indirectly by statistical approximation (low precision) | Results obtained directly by colony count (high precision) |
| Not readily adaptable for use in the field | Readily adapted for use in the field |
| Applicable to all types of water | Not applicable to turbid waters |
| Consumables readily available in most countries | Cost of consumables is high in many countries |
| May give better recovery of stressed or damaged organisms in some circumstances | No such difference observed |
FAQ
What is the use of membrane filter?
A membrane filter is a type of filter that uses a thin, porous membrane to separate particles or molecules from a liquid or gas. The membrane acts as a barrier that allows small molecules or particles to pass through, while larger particles are blocked.
Membrane filters are used in a wide range of applications, including water treatment, medical diagnostics, and industrial processes. In water treatment, membrane filters are used to remove bacteria, viruses, and other impurities from drinking water. In medical diagnostics, membrane filters are used to isolate and purify specific types of cells or molecules for analysis. In industrial processes, membrane filters are used to purify liquids and gases, such as in the production of beer, wine, and other beverages.
What does membrane filtration remove?
Increasingly, membrane techniques are used to remove bacteria, germs, particles, and natural organic material, which can lend colour, flavour, and odour to water and react with disinfectants to produce disinfection byproducts.
What is another name for membrane filtration?
Membrane filters, commonly referred to as membranes, are microporous sheets with particular pore size ratings. These are also known as microporous filters, screens, or sieves, and by the mechanism of surface capture, they retain microorganisms or particles larger than the size of their pores.
Why membranes are used for water treatment?
Membranes are used for water treatment because they are effective at removing impurities from water, including microorganisms, dissolved solids, and inorganic compounds. Membranes can also be used to remove specific contaminants, such as bacteria, viruses, and other microorganisms, from water.
Membranes have several advantages over other water treatment methods, such as chemical treatment or sedimentation. They do not require the use of chemicals, which can be harmful to the environment, and they use less energy than other methods. Membrane filtration also allows for a high degree of control over the quality of the treated water, and can be easily automated.
Additionally, membrane filtration can be used in a variety of settings, including large municipal water treatment plants, small-scale systems, and even portable treatment systems that can be used in remote locations or emergency situations. Membranes can be used in a variety of water treatment processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
Overall, membrane filtration is a highly efficient and cost-effective method for treating water, making it an attractive option for water treatment applications.
What is the main function of membrane filtration?
The main function of membrane filtration is to separate particles from a liquid or gas using a semipermeable membrane.
What are the types of membrane filtration?
The types of membrane filtration include microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
What are the advantages of membrane filtration?
Some advantages of membrane filtration include high efficiency, good selectivity, and low energy consumption.
What are the applications of membrane filtration?
Applications of membrane filtration include water treatment, biofiltration, industrial separations, and more.
What are the factors that affect the performance of membrane filtration?
Factors that affect the performance of membrane filtration include feed water quality, membrane material, pore size, and transmembrane pressure.
What are the common types of membrane materials used in membrane filtration?
Common types of membrane materials used in membrane filtration include cellulose acetate, polysulfone, polyamide, and polyvinylidene fluoride.
What is the mechanism of membrane filtration?
The mechanism of membrane filtration is based on the principle of size exclusion. The membrane has pores that are smaller than the particles to be separated, so the particles are unable to pass through the membrane while the liquid or gas is able to pass through.
What is the difference between microfiltration, ultrafiltration, and nanofiltration?
Microfiltration is used to remove particles larger than 0.1 microns, ultrafiltration is used to remove particles between 0.01 and 0.1 microns, and nanofiltration is used to remove particles between 0.001 and 0.01 microns.
What is the difference between reverse osmosis and ultrafiltration?
Reverse osmosis is a type of membrane filtration that removes dissolved salts and other small molecules, while ultrafiltration is used to remove particles between 0.01 and 0.1 microns.
What is the difference between dead-end and crossflow filtration?
Dead-end filtration is a method where the feed water flows into one end of the membrane and the permeate exits from the other end. In crossflow filtration, the feed water flows perpendicular to the membrane surface, allowing for continuous operation and lower fouling of the membrane.
How is the pore size of a membrane determined?
The pore size of a membrane is typically determined by measuring the size of the largest particle that can pass through the membrane.
How is the flux of a membrane determined?
The flux of a membrane is typically determined by measuring the amount of liquid or gas that can pass through the membrane over a given period of time.
How is the selectivity of a membrane determined?
The selectivity of a membrane is typically determined by measuring the ratio of the amount of a certain solute that can pass through the membrane to the amount of solvent that can pass through the membrane.
What are the common cleaning and maintenance procedures for membrane filtration?
Common cleaning and maintenance procedures for membrane filtration include chemical cleaning, backwashing, and membrane replacement.
What are the common challenges in membrane filtration and how can they be addressed?
Common challenges in membrane filtration include membrane fouling, membrane degradation, and poor selectivity. These challenges can be addressed through proper feed water treatment, regular cleaning and maintenance of the membrane, and careful selection of the membrane material and pore size to match the specific application. Additionally, using advanced technologies such as forward osmosis, and using chemicals like Hydrogen Peroxide, Chlorine and enzymes can also prevent membrane fouling.
- https://www.rpi.edu/dept/chem-eng/Biotech-Environ/Projects00/sterilize/filtration.html
- https://en.wikipedia.org/wiki/Microfiltration
- https://biologyreader.com/membrane-filtration-method.html
- https://www.slideshare.net/anupghimire/membrane-filtration-22725068
- https://www.slideshare.net/AkramHossain9/membrane-filtration-by-akram-hossain-food-and-process-engineering-hstu
- https://tuttnauer.com/blog/liquids-sterilization-by-filtration
- https://www.alfalaval.us/products/separation/membranes/what-is-membrane-filtration/