What is Lowenstein Jensen (LJ) Media?
- Lowenstein Jensen (LJ) Media is a solid agar-based medium that is widely used for the isolation and cultivation of Mycobacteria, particularly Mycobacterium tuberculosis, from clinical specimens. It falls under the category of egg-based media, which are solid media containing whole eggs or egg yolk, potato flour, salts, and glycerol.
- The original formulation of LJ medium was developed by Lowenstein and incorporated congo red and malachite green dyes to inhibit the growth of unwanted bacteria. Later, Jensen made modifications to the medium by adjusting the citrate and phosphate contents, removing the congo red dye, and increasing the concentration of malachite green. These modifications improved the selectivity and effectiveness of the medium for Mycobacteria isolation.
- Gruft further enhanced LJ medium by adding penicillin, nalidixic acid (FD053), and ribonucleic acid (RNA). Penicillin and nalidixic acid act as antimicrobics, preventing the growth of most contaminants surviving the decontamination process of the clinical specimen. RNA acts as a stimulant to increase the rate of isolation of Mycobacteria.
- The medium is designed to support the growth of a wide variety of Mycobacteria species and can also be used for niacin testing. It contains malachite green, which not only serves as an inhibitor of unwanted bacteria but also acts as a pH indicator. Gram-positive contaminants, such as Streptococci, cause a decrease in pH, resulting in the formation of a blue zone. Gram-negative bacilli can destroy the dye, leading to the formation of yellow zones. Additionally, proteolytic contaminants can cause localized or complete digestion of the medium.
- To maximize the effectiveness of LJ medium, it is recommended to inoculate and incubate each specimen in triplicate. This allows for the identification of saprophytes at room temperature (25°C) and the observation of pigmentation changes by photochromogenes and scotochromogenes alternately at 35°C in light and dark conditions, depending on the type of organism.
- Routine cultivation using LJ medium is typically carried out aerobically at 35°C, providing optimal conditions for the growth and isolation of Mycobacteria from clinical samples. However, it is important to note that glycerol should not be added to the medium if strains of Mycobacteria that are glycerophobic (intolerant to glycerol) or bovine in origin are to be cultured.
Lowenstein Jensen (LJ) Media Principle
The principle of Lowenstein Jensen (LJ) Media is based on its composition and the selective agents incorporated into the medium. LJ medium contains various components that provide essential nutrients and create a favorable environment for the growth of Mycobacteria while inhibiting the growth of contaminants.
L-Asparagine and Potato Flour serve as sources of nitrogen and vitamins, providing essential nutrients for the growth and metabolism of Mycobacteria. Monopotassium Phosphate and Magnesium Sulfate act as buffers and enhance organism growth.
Malachite green, along with Penicillin and Nalidixic acid in the Gruft method, plays a crucial role in preventing the growth of the majority of contaminants that may survive the decontamination process of the clinical specimen. These selective agents specifically target unwanted bacteria, allowing for the earliest possible growth of Mycobacteria.
Egg Suspension is an important component of LJ medium as it provides fatty acids and proteins that are essential for the metabolism of Mycobacteria. When heated, the egg albumin in the suspension coagulates, providing a solid surface for inoculation and the growth of Mycobacteria.
Glycerol, present in LJ medium, serves as a carbon source. It is favorable for the growth of the human-type tubercle bacillus, such as Mycobacterium tuberculosis, while being unfavorable to the bovine type.
Additionally, in the Gruft method, RNA is included as a stimulant to increase the rate of isolation of Mycobacteria. It helps in enhancing the growth and isolation of Mycobacteria from clinical specimens.
Overall, the principle of LJ medium lies in creating a selective environment with specific nutrients and selective agents that promote the growth of Mycobacteria while inhibiting the growth of contaminants, thereby facilitating the isolation and cultivation of Mycobacterium species, particularly Mycobacterium tuberculosis.

Lowenstein Jensen (LJ) Media Composition and Ingredients
Mineral salt solution
| Ingredients | Amount |
| Potassium dihydrogen phosphate anhydrous (KH2PO4) | 2.4 g |
| Magnesium sulphate anhydrous: (MgSo4.7H20) | 0.24 g |
| Magnesium citrate | 0.6 g |
| Asparagine | 3.6 g |
| Glycerol (reagent grade) | 12 ml |
| Distilled water | 600 ml |
Mix the components in the correct orderin distilled water through heating. Autoclave at 121°C for thirty minutes in order to clean. Cool to ambient temperature. The solution is stored for a long time and can be stored in adequate quantities inside the refrigerator.
Malachite green solution 2%
| Ingredients | Amount |
| Malachite green dye | 2.0 g |
| Distilled water | 100 ml |
*Dissolve the dye into the distillate water completely. Sort it out and place at room temperature in the freezer.
Homogenized whole eggs
- Take a fresh (those do not exceed 7 days old) eggs from a hen
- Cleanse the eggs by rubbing thoroughly with a toothbrush in soap and water.
- Allow the eggs to soak at least 30 minutes in soapy water.
- Wash eggs thoroughly in running water. Soak eggs in 70 percent ethanol for 15 minutes.
- Prior to handling the eggs, scrub them dry and rinse your hands with disinfectant.
- Crack the eggs using the beaker’s edge into a sterilized flask, and beat them with a sterilized blender in 30 second intervals to a minute.
Lowenstein Jensen (LJ) Media Preparation
To prepare Lowenstein Jensen (LJ) Media, follow these steps:
- Weigh 37.24 grams of LJ medium and suspend it in 600 ml of distilled water. If you are culturing bovine bacteria or other glycerophobic organisms, do not add glycerol at this stage.
- If necessary, heat the suspension to facilitate the complete dissolution of the medium.
- Sterilize the LJ medium by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
- Meanwhile, prepare aseptically 1000 ml of whole egg emulsion.
- Aseptically add the whole egg emulsion base and Gruft Mycobacterial Supplement (FD053) (if desired) to the sterilized LJ medium. Gently mix the components to obtain a uniform mixture.
- Distribute the LJ medium in sterile screw-capped tubes.
- Arrange the tubes in a slanted position to allow the medium to solidify at an angle.
- Coagulate and inspissate the medium by placing the tubes in an inspissator water bath or autoclaving them at 85°C for 45 minutes.
After following these steps, the Lowenstein Jensen (LJ) Media will be ready for use in the isolation and cultivation of Mycobacteria.
Method of Use of Lowenstein Jensen (LJ) Media
The method of using Lowenstein Jensen (LJ) Media can be outlined as follows:
- Specimen Collection: Collect the infectious material promptly and directly from the patient. Ensure that the specimen is protected from excessive heat and cold during transport to the laboratory. Refer to recommended references for specific guidelines on specimen collection.
- Inoculation: After decontamination and neutralization of the specimen, follow the test procedures recommended by the Centers for Disease Control (CDC) to inoculate the Lowenstein Jensen Media. Consult the listed references for detailed methods and protocols.
- Incubation: Place the inoculated LJ media in a carbon dioxide (CO2) atmosphere incubator set at 35-37ºC. Protect the media from light exposure throughout the incubation period. Initially, keep the caps of tubed media loosened for one week to allow the circulation of CO2 for the initiation of growth. After one week, tighten the caps to prevent dehydration of the media.
- Examination of Media: Begin examining the media within five to seven days of incubation and continue weekly for up to eight weeks. Look for the appearance of macroscopic growth on both plates and tubes.
- Macroscopic Growth Examination: Under suitable lighting conditions, observe the plates for the presence of visible colonies. Use a magnifying mirror to aid in the examination of the tubes for macroscopic growth. Record and describe the colony morphology on the first day growth is observed.
- Recording and Biochemical Identification: Consult appropriate references and guidelines for recording the number of colonies observed and for assistance in the biochemical identification of acid-fast bacilli.
Following these steps will enable the proper utilization of Lowenstein Jensen (LJ) Media for the cultivation and identification of Mycobacteria from clinical specimens. It is important to adhere to the recommended procedures and consult relevant references for accurate and reliable results.
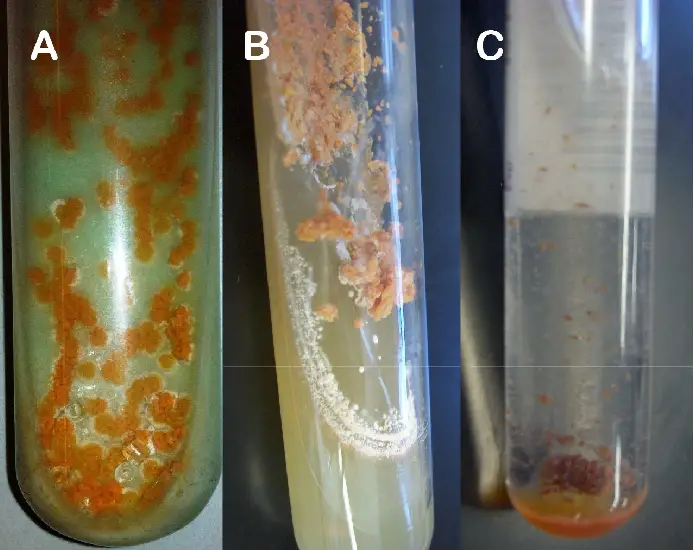
Result Interpretation of Lowenstein Jensen (LJ) Media
The interpretation of results obtained from Lowenstein Jensen (LJ) Media is as follows:
- Reading Time: Cultures should be examined and interpreted within 5 to 7 days after inoculation. After the initial reading, subsequent readings should be done once a week for up to 8 weeks to allow for the detection of slow-growing Mycobacteria.
- Colony Appearance: The typical colonies observed on LJ medium are non-pigmented, rough, and dry. These characteristics are often associated with the growth of Mycobacteria species.
- Green Color: The green color of the LJ medium is a result of the presence of malachite green, which serves as one of the selective agents in the medium. Malachite green helps prevent the growth of most other contaminants, ensuring that the observed colonies are likely Mycobacteria.
- Colony Development: Rapid-growing Mycobacteria species typically form mature colonies within 7 days of incubation. On the other hand, slow-growing Mycobacteria may require more than 7 days for their colonies to develop fully.
- Pigment Production: The color of the colonies can provide additional information about the Mycobacteria species. Nonchromogenic (NC) species typically produce white, cream, or buff-colored colonies. Chromogenic (Ch) species, on the other hand, can produce colonies in colors ranging from lemon, yellow, orange, to red.
By considering the reading time, colony appearance, green color of the medium, colony development, and pigment production, it is possible to interpret the results obtained from the growth of Mycobacteria on Lowenstein Jensen (LJ) Media. This interpretation can help in the identification and differentiation of different Mycobacteria species based on their growth characteristics.
Colony Characteristic of Different Microorganisms on Lowenstein Jensen (LJ) Media
| Organism | Colony Characteristic |
| Mycobacterium avium | smooth, nonpigmented colonies |
| Mycobacterium gordonae | smooth, yellow, orange colonies |
| Mycobacterium kansasii | photochromogenic, smooth to rough |
| Mycobacterium smegmatis | wrinkled,creamy white colonies |
| M. tuberculosis | granular, rough, warty, dry friable colonies |
Examination Schedule
Every culture should be examined for 72 hours following the inoculation to ensure that the liquid has evaporated completely, to seal the caps tight to stop drying out of the media and to identify contaminants. After that, the cultures are reviewed every week, or when this isn’t operationally feasible, at least three times, namely
- After one week, it is possible to identify mycobacteria rapidly growing that could be confused with M. tuberculosis.
- After 3-4 weeks, you can detect positive culture from M. tuberculosis, as well with other mycobacteria that slow-growing which could be harmless saprophytes or pathogens
- After 8 weeks, it is possible to identify very slow-growing mycobacteriasuch as M. tuberculosis. Before concluding the culture is negative.
Quality Control
Quality control of Lowenstein Jensen (LJ) Media involves assessing various aspects of its appearance, gelling, color, clarity, and cultural response. The following information can be used to understand the quality control measures:
- Appearance: The LJ medium should have a greenish blue to peacock blue homogeneous appearance. It should be in the form of a free-flowing powder.
- Gelling: The LJ medium should gel properly when prepared. It should form a coagulated, pale bluish-green colored, opaque, and smooth slant after inspissation.
- Color and Clarity of Prepared Medium: The mixture of sterile basal medium and whole egg emulsion, when inspissated, should result in a pale bluish-green colored, opaque, and smooth slant.
- Cultural Response: The LJ medium should be tested for its cultural response using specified strains of Mycobacteria. Incubation is done at 35-37ºC for 2-4 weeks in the presence of 5-10% carbon dioxide, along with the addition of egg emulsion base.
- Mycobacterium avium ATCC 25291: The cultural response should show luxuriant growth with smooth, nonpigmented colonies.
- Mycobacterium gordonae ATCC 14470: The cultural response should exhibit luxuriant growth with smooth, yellow or orange colonies.
- Mycobacterium kansasii ATCC 12478: The cultural response should demonstrate luxuriant growth, and the colonies can be photochromogenic, ranging from smooth to rough.
- Mycobacterium smegmatis ATCC 14468: The cultural response should show luxuriant growth with wrinkled, creamy white colonies.
- Mycobacterium tuberculosis H37RV ATCC 25618: The cultural response should exhibit luxuriant growth with granular, rough, warty, dry, and friable colonies.
These specified strains are used as controls to assess the expected growth and characteristics of Mycobacteria on LJ media.
By evaluating the appearance, gelling, color, clarity, and cultural response according to the specified criteria, the quality of Lowenstein Jensen (LJ) Media can be effectively monitored and controlled to ensure consistent and reliable performance in the isolation and identification of Mycobacteria.
Lowenstein Jensen (LJ) Media Uses
Lowenstein Jensen (LJ) Media has several important uses in the field of microbiology, specifically for the diagnosis and characterization of Mycobacterial infections. These uses include:
- Diagnosis of Mycobacterial Infections: LJ medium is commonly used for the isolation and cultivation of Mycobacteria from clinical specimens, particularly for the diagnosis of Mycobacterial infections. The medium provides an environment that supports the growth of Mycobacteria, allowing for their isolation and subsequent identification.
- Antibiotic Susceptibility Testing: LJ medium can also be utilized for testing the antibiotic susceptibility of Mycobacterial isolates. By inoculating the isolates onto LJ medium, the growth patterns and responses to different antibiotics can be observed and analyzed, aiding in the determination of the appropriate treatment options.
- Differentiation of Mycobacterial Species: LJ medium is valuable for differentiating various species of Mycobacteria based on colony morphology, growth rate, biochemical characteristics, and microscopy. Different species exhibit unique growth patterns, colony appearances, and responses to specific biochemical tests. These characteristics can be observed and recorded when isolates are cultured on LJ medium, helping in the identification and differentiation of Mycobacterial species.
Overall, Lowenstein Jensen (LJ) Media serves as a versatile tool in the laboratory for the diagnosis of Mycobacterial infections, testing antibiotic susceptibility, and differentiating different species of Mycobacteria based on their growth characteristics and biochemical profiles. Its use contributes to accurate identification and characterization of Mycobacterial isolates, which is crucial for appropriate patient management and control of Mycobacterial infections.
Limitations of Lowenstein Jensen (LJ) Media
Lowenstein Jensen (LJ) Media, despite its usefulness in the isolation and identification of Mycobacteria, has certain limitations that should be considered. These limitations include:
- Incomplete Identification: While LJ medium provides a favorable environment for the growth of Mycobacteria, it does not provide comprehensive identification of the isolated strains. It is recommended to perform additional biochemical and/or serological tests on colonies from pure culture to achieve complete identification of the Mycobacterial species.
- CO2 Requirement: LJ media typically requires incubation in a 5-10% carbon dioxide (CO2) atmosphere to optimize the recovery of Mycobacteria. However, it is important to note that Mycobacteria may not be well recovered from candle extinction jars, and thus alternative methods or equipment may be necessary for optimal growth.
- Negative Culture Results: A negative culture result on LJ media does not completely rule out an active Mycobacterial infection. It is possible that the clinical specimen may contain a low number of Mycobacteria, making them difficult to detect in culture. Additional diagnostic methods, such as molecular techniques, may be required for a more definitive diagnosis.
- Nutritional Variation: LJ media may encounter strains of Mycobacteria that grow poorly or fail to grow altogether due to nutritional variations. This limitation highlights the importance of conducting further tests for confirmation of Mycobacterium species and the need for additional culture media or methods to support the growth of these challenging strains.
- Photosensitivity: LJ media containing malachite green should be protected from all sources of light. Malachite green is highly photosensitive, and exposure to light can lead to its degradation or loss of inhibitory properties. Proper storage and handling of LJ media are crucial to maintain its effectiveness.
Considering these limitations, it is essential to employ a combination of culture-based methods, additional tests, and alternative media to overcome challenges and ensure accurate detection and identification of Mycobacteria in clinical specimens.
FAQ
What is Lowenstein Jensen (LJ) Media?
Lowenstein Jensen (LJ) Media is a solid agar-based medium commonly used for the isolation and cultivation of Mycobacteria, particularly Mycobacterium tuberculosis, from clinical specimens.
What is the composition of LJ Media?
LJ Media consists of ingredients such as L-asparagine, potato flour, monopotassium phosphate, magnesium sulfate, glycerol, and selective agents like malachite green. The exact composition may vary slightly depending on the specific formulation.
How does LJ Media inhibit the growth of contaminants?
LJ Media contains selective agents like malachite green, which prevent the growth of most contaminants surviving the decontamination process of the clinical specimen, while encouraging the growth of Mycobacteria.
What is the recommended incubation temperature and atmosphere for LJ Media?
LJ Media is typically incubated at 35-37°C in a carbon dioxide (CO2) atmosphere. This helps create optimal conditions for the growth of Mycobacteria.
How long should LJ Media be incubated?
Cultures on LJ Media should be read within 5 to 7 days after inoculation and once a week thereafter for up to 8 weeks to allow for the detection of slow-growing Mycobacteria.
Can LJ Media be used for antibiotic susceptibility testing?
Yes, LJ Media can be used for antibiotic susceptibility testing of Mycobacterial isolates. The growth patterns and responses of the isolates to different antibiotics can be observed and analyzed on LJ Media.
Can LJ Media differentiate different species of Mycobacteria?
Yes, LJ Media can aid in the differentiation of different species of Mycobacteria based on colony morphology, growth rate, biochemical characteristics, and microscopy.
What are the limitations of LJ Media?
Some limitations of LJ Media include the need for additional tests for complete identification of isolated strains, the requirement for a CO2 atmosphere for optimal recovery of Mycobacteria, the possibility of poor growth or failure to grow for certain strains, and the photosensitivity of malachite green in the medium.
How should LJ Media be stored?
LJ Media should be stored in a cool and dry place, protected from light to prevent degradation or loss of the selective agents, particularly malachite green.
Is LJ Media the only medium used for Mycobacteria culture?
No, LJ Media is one of the commonly used media for Mycobacteria culture, but there are other media available as well, such as Middlebrook 7H10 agar, Middlebrook 7H11 agar, and liquid media like Middlebrook 7H9 broth, which can be used depending on the specific requirements of the laboratory or the type of Mycobacteria being cultured.
References
- https://exodocientifica.com.br/_technical-data/M162.pdf
- Laboratory services in Tuberculosis Control, Part III: Culture, World Health Organization
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/LJMedia.htm
- http://himedialabs.com/td/m162.pdf
- https://www.thermofisher.com/order/catalog/product/R10100
- https://bioactiva.com/pub/media/sebwite/productdownloads//l/o/lowenstein-jensen-tube-maunal.pdf
- https://www.biotrend.com/en/buy/cat-lowenstein-jensen-lj-agar-selective-5527.html
- https://hardydiagnostics.com/x22
- https://microbeonline.com/preparation-uses-lowenstein-jensen-lj-medium/
- https://laboratoryinfo.com/lj-medium/