What is Implantation?
- Implantation, also known as nidation, is a crucial stage in the development of mammals where the blastocyst, a hollow ball of cells, attaches and invades the wall of the female’s uterus. This process allows the embryo to establish a connection with the mother’s body and receive the necessary oxygen and nutrients for further growth and development. Successful implantation is a significant milestone, as it marks the beginning of pregnancy.
- In women, the presence of increased levels of human chorionic gonadotropin (hCG) in a pregnancy test is an indication of an implanted embryo. This hormone is produced by the developing placenta and serves as a marker for pregnancy.
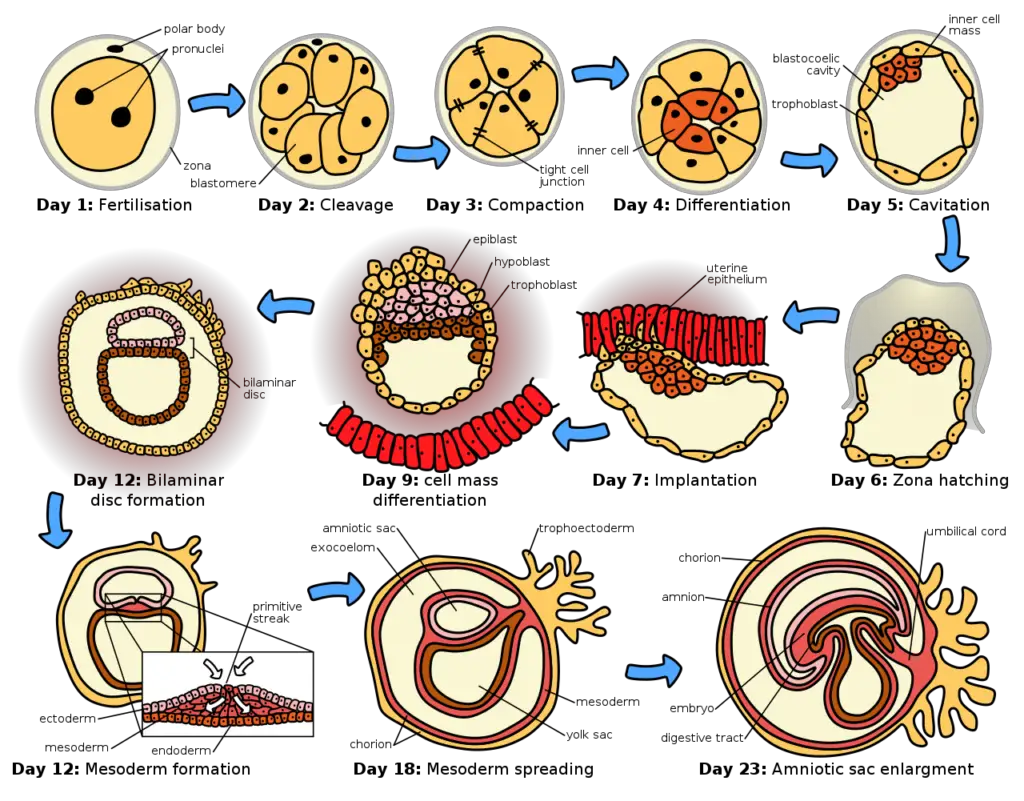
- The process of implantation involves several stages, although the specific details can vary among different mammalian species. The five recognized stages of implantation are migration and hatching, pre-contact, attachment, adhesion, and invasion. These stages are similar across various species, except for the two pre-implantation stages that occur before placentation.
- In humans, implantation begins after the blastocyst has hatched, typically around four to five days after fertilization. During the first week, the blastocyst attaches superficially to the uterine endometrium. By the end of the second week, the process of implantation is completed.
- The intricate process of implantation involves a complex interaction between the blastocyst and the uterine lining. Specialized trophoblast cells play a crucial role in this process, ensuring the establishment of a stable connection between the developing embryo and the maternal tissues.
- Implantation in humans is a highly regulated and delicate process. Any disruptions or abnormalities during implantation can lead to implantation failure or early pregnancy loss. Understanding the mechanisms and factors involved in successful implantation is essential for reproductive health and assisted reproductive technologies.
- Overall, implantation is a critical step in mammalian embryonic development, enabling the embryo to access the necessary resources for growth and signaling the initiation of pregnancy.
Time of Implantation
- The time of implantation refers to the specific period when the blastocyst, after its journey through the fallopian tubes, reaches the uterus and attaches to the endometrium. The process of implantation typically occurs within a certain timeframe, although the exact timing can vary among individuals.
- After the blastocyst enters the uterine cavity, it usually remains there for approximately 1 to 3 days before initiating the process of implantation. During this time, the blastocyst undergoes changes and prepares itself for attachment to the endometrial lining.
- Around the 6th to 7th day after fertilization, the blastocyst comes into contact with the endometrial wall. This initial contact is an important step in the process of implantation, as it sets the stage for further interactions between the blastocyst and the endometrium.
- Over the next few days, the blastocyst continues to interact with the endometrial tissue and undergoes further changes to establish a strong connection. By the 12th day after fertilization, the blastocyst becomes properly implanted in the endometrium, firmly anchoring itself for further development.
- It’s important to note that the timing of implantation can vary among individuals and may be influenced by various factors, including the specific characteristics of the blastocyst and the receptivity of the endometrium. The process of implantation is highly regulated and requires a synchronized interplay between the developing embryo and the maternal tissues.
- Understanding the timing of implantation is crucial in reproductive medicine, as it provides insights into the optimal window for successful implantation and the potential causes of implantation failure. It also has implications for assisted reproductive technologies, such as in vitro fertilization (IVF), where the timing of embryo transfer is carefully planned to maximize the chances of successful implantation.
- In summary, the time of implantation refers to the period when the blastocyst attaches to the endometrial lining after its arrival in the uterus. While the blastocyst typically spends 1 to 3 days in the uterine cavity before initiating implantation, it usually establishes proper implantation around the 12th day after fertilization. However, it’s important to recognize that individual variations can occur, and the process of implantation is a complex and highly regulated event in reproductive biology.
Site of Implantation
- The site of implantation refers to the specific location within the uterus where the blastocyst attaches and becomes implanted. The uterus provides a suitable environment for the development of the embryo, and the site of implantation plays a crucial role in the successful establishment of pregnancy.
- In most cases, the blastocyst tends to implant in the fundus of the uterus. The fundus refers to the upper portion of the uterus, which is the part farthest away from the cervix. This region offers a favorable environment for implantation due to its rich blood supply and optimal conditions for nourishing the developing embryo.
- However, it’s important to note that the blastocyst can potentially implant in other areas of the uterus as well. The body of the uterus, which is the central part between the fundus and the cervix, is another possible site for implantation. The decision of where the blastocyst implants can depend on various factors, including the specific characteristics of the embryo and the receptivity of the endometrium.
- In some cases, ectopic pregnancies may occur, where the blastocyst implants outside the uterus. This can lead to serious complications and requires medical attention. Ectopic pregnancies commonly occur in the fallopian tubes, but they can also happen in other locations, such as the ovaries or abdominal cavity.
- The site of implantation is influenced by complex interactions between the blastocyst and the endometrium. The endometrium undergoes cyclic changes throughout the menstrual cycle, becoming more receptive to implantation during a specific phase known as the receptive or “implantation window.” This window of receptivity typically occurs around 6 to 10 days after ovulation, coinciding with the arrival of the blastocyst in the uterus.
- Understanding the site of implantation is essential in reproductive medicine and assisted reproductive technologies. In procedures like in vitro fertilization (IVF), the embryo transfer is carefully performed to place the blastocyst at the optimal site within the uterus for successful implantation.
- In conclusion, the site of implantation refers to the specific location within the uterus where the blastocyst becomes attached and implanted. While the fundus of the uterus is the most common site for implantation, the blastocyst can potentially implant in other areas of the uterus as well. The site of implantation is influenced by various factors, and it plays a critical role in the establishment and development of a healthy pregnancy.
Implantation stages
Implantation is a complex process that involves a series of stages in the embryonic development of mammals. These stages are crucial for the successful attachment and invasion of the blastocyst into the uterine wall. The five recognized stages of implantation are migration and hatching, pre-contact, attachment, adhesion, and invasion. Let’s explore each of these stages:
- Migration and Hatching: After reaching the uterus, the blastocyst undergoes migration within the uterine cavity. It moves and floats freely before hatching from the protective zona pellucida, a glycoprotein shell that surrounded the early embryo during its development in the fallopian tube.
- Pre-Contact: During this stage, the blastocyst approaches the receptive endometrium, which is the lining of the uterus that has reached its optimal state for implantation. The blastocyst starts to make contact with the endometrial surface but does not yet attach.
- Attachment: In this stage, the blastocyst firmly attaches to the endometrial lining. Specialized cells called trophoblasts, derived from the outer layer of the blastocyst, interact with the endometrium. These trophoblasts initiate the formation of the placenta, which will provide oxygen and nutrients to the developing embryo.
- Adhesion: Once attached, the blastocyst further adheres to the endometrium, establishing a stronger connection. This adhesion is mediated by specific molecules and cell-to-cell interactions between the trophoblasts and the endometrial cells.
- Invasion: The final stage of implantation involves the invasion of the trophoblasts into the endometrial tissue. This invasion allows the blastocyst to establish a close relationship with the maternal blood vessels and facilitate the exchange of nutrients and waste products. The depth and extent of trophoblast invasion vary among species, and it is a highly regulated process to ensure proper placental development.
It’s important to note that these stages occur within a limited timeframe known as the window of implantation. The uterus is only receptive to implantation during this specific period, which is influenced by hormonal changes and the cyclic nature of the endometrial lining.
Understanding the stages of implantation is vital for reproductive medicine and fertility treatments. Assisted reproductive technologies, such as in vitro fertilization (IVF), aim to optimize the timing of embryo transfer to align with the window of implantation, increasing the chances of successful implantation and pregnancy.
In summary, the process of implantation in mammals involves five recognized stages: migration and hatching, pre-contact, attachment, adhesion, and invasion. These stages are essential for the blastocyst to establish a secure connection with the endometrium and develop into a healthy pregnancy. The timing and success of implantation depend on various factors, including the receptivity of the uterus and the intricate interactions between the embryo and the maternal tissues.
Migration and hatching
Migration and hatching are two important stages in the process of implantation during embryonic development. Let’s explore these stages in more detail:
- Migration of the Zygote: After fertilization occurs in the fallopian tube, the zygote undergoes migration towards the uterus. Cilia present in the lining of the fallopian tube aid in the movement of the zygote. During this migration, the zygote undergoes multiple cell divisions, resulting in the formation of a compacted ball of cells called a morula.
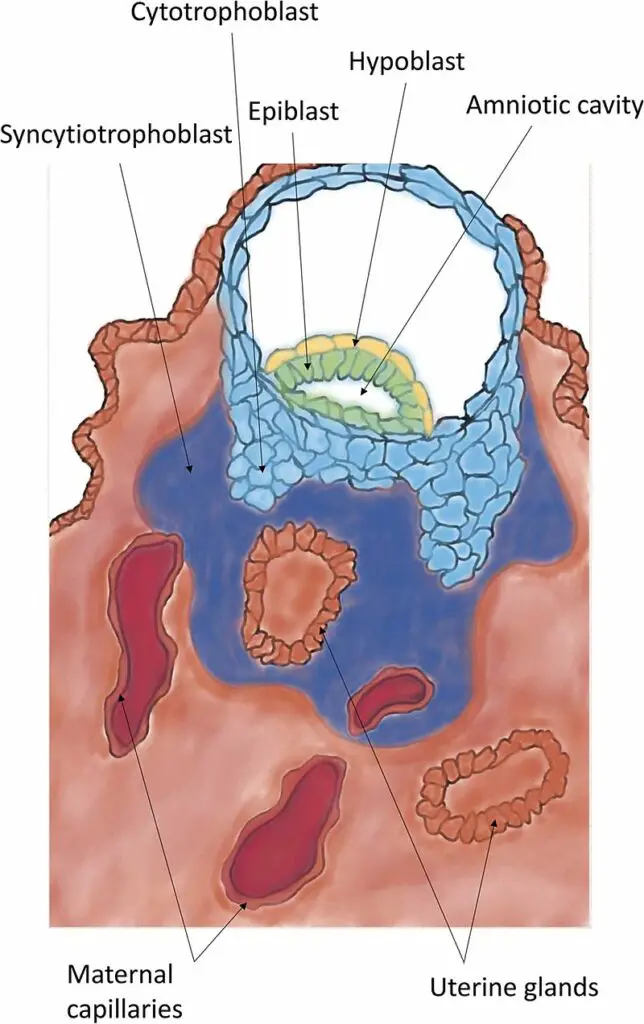
- Migration of the Trophoblast: As the morula enters the uterus, it undergoes further changes. A cavity called the blastocoel forms within the morula, transforming it into a blastocyst. The blastocyst consists of an inner cell mass that will develop into the embryo and an outer cell layer called the trophoblast, which will give rise to the extraembryonic membranes.
- Zona Hatching: The blastocyst remains enclosed in a protective egg-coat called the zona pellucida. In order to implant into the uterine wall, the blastocyst must shed this covering. This process is known as zona hatching. The breakdown of the zona pellucida is facilitated by lytic factors present in the uterine cavity and factors produced by the blastocyst itself. Proteases stimulated by various growth factors play a significant role in the dissolution of the zona pellucida.
- Apposition: Once the zona pellucida has been sufficiently dissolved, the blastocyst can initiate the apposition stage of implantation. Apposition refers to the physical contact between the blastocyst and the receptive endometrium of the uterus. This contact is essential for the subsequent stages of implantation to take place.
During these stages, various molecular regulators and cytokines are involved in the hatching process. Proteases, stimulated by growth factors, contribute to the breakdown of the zona pellucida. Cytokines, including pro-inflammatory and anti-inflammatory types, play important roles in implantation and other stages of pregnancy. Leukemia inhibitory factor (LIF), a pro-inflammatory cytokine expressed in the endometrium, is involved in adhesion and invasion.
In certain cases, assisted reproductive technologies may utilize assisted zona hatching. This involves artificially piercing the zona pellucida to facilitate hatching, increasing the chances of successful implantation.
In summary, migration and hatching are crucial stages in the process of implantation. Migration involves the movement of the zygote towards the uterus, while hatching refers to the shedding of the zona pellucida by the blastocyst. These stages are essential for the blastocyst to establish contact with the receptive endometrium and initiate the subsequent stages of implantation.
Apposition
- Apposition is an important stage in the process of implantation, following the hatching of the blastocyst. Let’s delve deeper into the concept:
- Apposition refers to the initial loose contact or connection established between the blastocyst and the endometrium of the uterus. This contact occurs at specific sites in the endometrium, often in small crypts or invaginations. The breakdown of the zona pellucida allows the trophoblast layer of the blastocyst to directly contact the underlying endometrium.
- During apposition, the inner cell mass, also known as the embryoblast, aligns itself closest to the decidua, which is the specialized uterine lining. If the inner cell mass is not initially aligned with the decidua during apposition, it has the ability to rotate freely within the trophoblast layer until proper alignment is achieved.
- It is important to note that apposition represents a weak interaction between the trophectoderm (the outer cell layer of the blastocyst) and the uterine epithelium. This connection is unstable and susceptible to shear stress. This reversible interaction allows for the repositioning of the blastocyst within the uterus if necessary.
- Apposition sets the stage for further stages of implantation, such as attachment, adhesion, and invasion, to take place. It serves as the initial point of contact between the developing embryo and the maternal tissues, paving the way for the establishment of a firm attachment and subsequent invasion into the endometrium.
- In summary, apposition marks the first loose contact between the blastocyst and the endometrium following zona hatching. It occurs at specific sites in the endometrium, where the trophoblast of the blastocyst makes direct contact with the underlying endometrium. Apposition is a reversible and unstable interaction that sets the foundation for subsequent stages of implantation to occur.
Adhesion
- Adhesion is a crucial stage in the process of implantation, where the attachment between the trophoblast cells of the blastocyst and the endometrium becomes stronger. Let’s explore this stage in more detail:
- During adhesion, the trophoblast cells penetrate the endometrium, establishing a more secure attachment compared to the previous apposition stage. This penetration involves the extension of trophoblast cell protrusions into the endometrial tissue.
- Microvilli present on the surface of the trophoblast cells play a significant role in the adhesion process. These microvilli, along with binding fiber connections, laminin, collagen type IV, and integrins, facilitate the adhesion of the trophoblast to the endometrium.
- The mucin-16 protein, also known as MUC-16, is expressed on the apical surface of the uterine epithelia. It acts as a barrier preventing the blastocyst from implanting in undesired locations on the epithelium. The removal of MUC-16 during the formation of pinopodes (protrusions on the endometrial surface) has been observed to promote trophoblast invasion in vitro.
- The specific molecules involved in the initial interaction between the trophoblast and the endometrial epithelia have not been fully identified. However, researchers have proposed that MUC1, a glycosylated protein belonging to the mucin family, may play a role. MUC1 is expressed on the apical surface of endometrial epithelial cells during the window of implantation in humans. It exhibits carbohydrate moieties that act as ligands for L-selectin, a cell adhesion molecule present on trophoblast cells. Studies have indicated that L-selectin interacts with its ligands to mediate the apposition of the blastocyst to the uterine epithelium.
- In summary, adhesion represents a stronger attachment between the trophoblast cells and the endometrium compared to apposition. It involves the penetration of trophoblast cells into the endometrial tissue, facilitated by microvilli and various molecules such as MUC1 and L-selectin. Adhesion is a critical step in the implantation process, setting the stage for subsequent stages, including invasion, to occur.
Invasion
- Invasion is a critical stage in the process of implantation where the blastocyst further establishes itself into the endometrium. Let’s delve into the key aspects of this stage:
- During invasion, trophoblast cells, which have adhered to the endometrium, continue to proliferate and penetrate deeper into the endometrial tissue. This penetration is facilitated by the activity of gelatinase A and B, enzymes involved in breaking down the extracellular matrix.
- The primary objective of trophoblast invasion is to reach the maternal blood supply, allowing the establishment of a connection for fetal blood flow. As trophoblasts penetrate deeper, they undergo terminal differentiation and form a multinucleated tissue called the syncytiotrophoblast. The syncytiotrophoblast lies between the blastocyst and the endometrium, while the cytotrophoblast surrounds the inner cell mass.
- When the syncytiotrophoblast reaches the basal membrane beneath the decidual cells, it dislodges them, allowing further invasion into the uterine stroma. This dislodging is accomplished by degrading cell adhesion molecules and the associated extracellular matrix. The syncytiotrophoblast secretes tumor necrosis factor-alpha, which inhibits the expression of cell adhesion molecules and beta-catenin. The degradation of the extracellular matrix is carried out by various enzymes, including metalloproteinases and serine proteases.
- As the syncytiotrophoblast invades the endometrium, it carries the embryo with it, eventually coming into contact with maternal blood vessels. This interaction leads to the formation of chorionic villi, marking the beginning of placentation.
- After invasion, the breach created in the uterine epithelium by the blastocyst’s entry is sealed by a fibrin plug, which consists of a coagulation of blood clot and cellular debris.
- In addition to invading the endometrium, extravillous trophoblasts migrate into the myometrium of the mother’s uterus. These trophoblasts remodel the spiral arteries, ensuring improved and secured maternal blood flow to support the growing embryo. They may also stabilize uterine veins, facilitating drainage of fetal blood and metabolic wastes.
- During invasion, the blastocyst secretes various factors with multiple functions. These secretions include autocrine factors that stimulate the blastocyst itself to further invade the endometrium. They also loosen decidual cells, prevent rejection of the embryo by the mother’s immune system, trigger final decidualization, and prevent menstruation.
- Immunosuppressive agents are secreted by the blastocyst to prevent the mother’s immune system from rejecting the embryo. These agents include platelet-activating factor, human chorionic gonadotropin, early pregnancy factor, prostaglandin E2, interleukin-1 alpha, interleukin-6, interferon-alpha, leukemia inhibitory factor, and colony-stimulating factor.
- Human chorionic gonadotropin (hCG), one of the secreted factors, not only acts as an immunosuppressive agent but also signals to the mother’s body that she is pregnant. This prevents luteolysis of the corpus luteum, sustaining its function and preventing menstruation.
- Various other factors are secreted by the blastocyst during invasion, including histamine-releasing factor, tissue plasminogen activator and its inhibitors, estradiol, β1-integrins, fibroblast growth factor, CYTL1, transforming growth factor alpha, inhibin, and preimplantation factor.
- In summary, invasion is a crucial stage where trophoblast cells proliferate, penetrate the endometrium, and establish a deeper connection. This process involves the formation of syncytiotrophoblast, degradation of cell adhesion molecules and the extracellular matrix, and the eventual formation of chorionic villi. The invasion is accompanied by the secretion of various factors that support the implantation process, prevent rejection, and sustain the early pregnancy.
Process of Implantation of the Blastocyst in the Uterus
The process of implantation of the blastocyst in the uterus involves several stages that are essential for successful pregnancy. Let’s explore these stages based on the provided information:
- Formation of the Morula: After fertilization occurs, the zygote undergoes rapid cell division, forming a solid ball of cells called the morula. This transformation takes place within the fallopian tube.
- Transport to the Uterus: The morula takes approximately 3 to 5 days to travel through the fallopian tube and reach the uterine cavity. This transport is facilitated by a combination of fluid currents created by epithelial secretions and the beating motion of cilia lining the fallopian tube, which propel the morula toward the uterus. Weak contractions of the fallopian tube may also aid in this process.
- Transformation into a Blastocyst: Within the uterus, the morula further develops into a blastocyst, which consists of approximately 100 cells. The blastocyst is a fluid-filled structure with an outer layer of cells called the trophoblast and an inner cell mass.
- Implantation: The blastocyst spends 3 to 6 days freely floating in the uterine cavity before it becomes implanted in the uterine endometrium. During this time, the blastocyst relies on the nutritive secretions of the uterine endometrium, often referred to as “uterine milk.”
- Trophoblast Action: Implantation occurs due to the activity of the trophoblast cells on the surface of the blastocyst. These trophoblast cells secrete proteolytic enzymes that digest and liquefy the adjacent cells of the uterine endometrium. This process allows the blastocyst to penetrate and anchor itself to the endometrium. Some of the fluid and nutrients released during this action are actively transported by the trophoblast cells into the blastocyst, providing additional nourishment for its growth.
- Proliferation and Placenta Formation: Once implantation takes place, the trophoblast cells and adjacent cells from both the blastocyst and the uterine endometrium rapidly proliferate. This proliferation leads to the formation of the placenta and various membranes of pregnancy, which play crucial roles in supporting fetal development and maintaining pregnancy.
Receptivity of Uterus
The receptivity of the uterus plays a crucial role in the process of implantation. It involves various changes in the endometrial cells and tissues that prepare the uterus to receive and support the conceptus. Here are the key aspects of uterine receptivity based on the provided information:
- Plasma Membrane Transformation: Receptivity involves changes in the endometrial cells known as plasma membrane transformation. These changes include the formation of pinopodes, which are mushroom-like protrusions from the apical cell membrane of uterine epithelial cells. Pinopodes are ultrastructural markers of receptivity and are fully formed during the window of implantation, typically between days 19 and 21 of gestational age.
- Decidualization: Decidualization refers to the process of the endometrium becoming prepared for implantation. It includes an increase in endometrial thickness, vascularization, and growth of the glands. The endometrium also produces decidual cells, which form a new layer called the decidua. The decidua plays a crucial role in supporting and nourishing the developing embryo.
- Window of Implantation: The window of implantation is a limited timeframe during which the endometrium is optimally receptive for the attachment of the blastocyst. In humans, this window occurs between days 20 and 24 of the secretory phase of the menstrual cycle, when luteinizing hormone levels are at their peak. The window of implantation lasts only 24 to 36 hours, emphasizing the importance of timing for successful implantation.
- Receptor-Ligand Interactions: During the window of implantation, there is significant communication between the blastocyst and the endometrium. Receptor-ligand interactions, specifically involving integrin-matrix and proteoglycan receptors, play a role in this communication. Proteoglycan receptors are found on the surface of the decidua, while the blastocyst’s trophoblast cells have corresponding proteoglycans. These interactions contribute to the adhesion and invasion of the blastocyst into the endometrium.
- Pinopodes: Pinopodes, which are formed during the window of implantation, aid in the receptivity of the uterus. They enhance the absorption of uterine fluid, reducing the volume of the uterus and bringing the blastocyst closer to the endometrium. Pinopodes also facilitate direct contact and adherence between the blastocyst and the uterine epithelial cells, promoting implantation.
- Decidualization and Embryonic Influence: Decidualization not only prepares the endometrium for implantation but also responds to signals from the developing embryo. Factors released by the blastocyst trigger the final formation of decidual cells and further changes in the endometrium. This interaction highlights the co-dependence between the viability of the embryo and the receptivity of the uterus.
Clinical significance of Implantation
The clinical significance of implantation encompasses several aspects that are important to consider. Here are the key points derived from the provided information:
- Implantation Failure: Implantation failure is a significant contributor to unsuccessful pregnancies. Approximately 85% of failed pregnancies can be attributed to implantation failure, making it a major concern in reproductive medicine. In two-thirds of cases, inadequate uterine receptivity is the cause, while problems with the embryo itself account for the remaining third. Implantation failure is also a common reason for the failure of in vitro fertilization (IVF) procedures.
- Causes of Inadequate Uterine Receptivity: Inadequate uterine receptivity can be caused by various factors, including abnormal cytokine and hormonal signaling, as well as epigenetic alterations. Identifying the underlying causes of implantation failure is crucial for optimizing endometrial receptivity and improving pregnancy rates. Evaluation of implantation markers can aid in predicting pregnancy outcomes and detecting occult implantation deficiencies. Advanced technologies like organ-on-a-chip systems and endometrium organoids have been developed to better understand and model the functioning of the endometrium in relation to implantation.
- Interventions to Improve Implantation: In cases of recurrent implantation failure, certain interventions can be employed to enhance the chances of successful implantation. For women with more than three implantation failures in assisted reproduction, the use of low molecular weight heparin as an adjunct has shown promising results, improving the live birth rate by approximately 80%. Additionally, luteal phase support, which includes the administration of progesterone and human chorionic gonadotropin (hCG), can be employed to improve the likelihood of successful implantation.
- Zinc Deficiency: Zinc plays a crucial role in pre-conception and successful pregnancy. Zinc deficiency can lead to incompetent blastocyst development. During fertilization, zinc is released in a zinc spark, which promotes changes in the egg, including the hardening of the zona pellucida to prevent polyspermy. Ensuring adequate zinc levels is important for optimal reproductive outcomes.
- Implantation Bleeding: Implantation bleeding refers to light vaginal bleeding or spotting that can occur in early pregnancy between 7 and 14 days after fertilization. It is caused by the blastocyst penetrating the lining of the uterus during implantation. Implantation bleeding is typically a small amount of blood and can be accompanied by symptoms such as cramping, nausea, breast tenderness, and headaches. Distinguishing implantation bleeding from menstrual bleeding involves considering factors such as color, clotting, strength, and duration of flow.