What is Human Immunodeficiency Virus (HIV)?
- Human Immunodeficiency Virus (HIV) is a significant retrovirus that plays a critical role in the development of Acquired Immunodeficiency Syndrome (AIDS). Recognized as one of the most severe epidemics globally, AIDS was first identified in Los Angeles in 1981 through the emergence of five cases of Pneumocystis jirovecii pneumonia, primarily among homosexual men and individuals who used intravenous drugs. These early observations marked the beginning of an urgent investigation into the cause of this alarming syndrome.
- In 1983, researchers led by Luc Montagnier at the Pasteur Institute in Paris successfully isolated a retrovirus from a West Asian patient suffering from persistent generalized lymphadenopathy. This virus was initially named Lymphadenopathy-associated Virus (LAV). A year later, Robert Gallo and his team at the National Institutes of Health in the United States independently identified a retrovirus in patients with AIDS, naming it Human T-cell Lymphotropic Virus-III (HTLV-III). In 1986, the International Committee on Virus Nomenclature unified these findings by assigning the name Human Immunodeficiency Virus, or HIV, to the virus responsible for AIDS.
- HIV is categorized within the lentivirus subgroup of the Retroviridae family. This classification includes other slow-acting viruses responsible for diseases such as visna/maedi in sheep and infectious anemia in horses and goats. Within the context of human health, HIV is classified into two primary types: HIV-1, the most prevalent and virulent strain associated with global AIDS cases, and HIV-2, which has been primarily identified in certain regions of West Africa.
- HIV targets the body’s immune system, specifically attacking CD4+ T cells, which play an essential role in the immune response. The virus uses these cells to replicate and spread throughout the body, leading to a gradual decline in immune function. Consequently, individuals infected with HIV become increasingly susceptible to opportunistic infections and diseases, which are hallmark characteristics of AIDS.
- The modes of transmission for HIV are well-documented, primarily occurring through unprotected sexual contact, sharing of contaminated needles, and transmission from mother to child during childbirth or breastfeeding. Understanding these transmission routes is vital for implementing effective prevention strategies.
- HIV infection progresses through several stages, beginning with acute HIV infection, followed by a clinical latency stage, where the virus remains inactive. Without treatment, this latent phase can last for years before advancing to AIDS. It is crucial to note that the presence of HIV does not always equate to an AIDS diagnosis; AIDS is defined by specific clinical criteria, including CD4+ T cell counts falling below a critical threshold and the occurrence of certain opportunistic infections.
Classification of HIV
Human Immunodeficiency Virus (HIV) is classified into two primary types: HIV-1 and HIV-2. Both types belong to the Retroviridae family, specifically under the genus Lentivirus. These viruses exhibit significant diversity, which is crucial for understanding their epidemiology, transmission dynamics, and treatment options.
- HIV Types:
- HIV-1: This is the more prevalent and widely studied type, responsible for the majority of global HIV infections. It is further categorized into several groups:
- Group M (Major): This group accounts for the vast majority of HIV infections worldwide and is the primary driver of the HIV/AIDS pandemic. Within Group M, there are numerous subtypes (also known as clades), designated by letters A through K, reflecting a high level of genetic diversity. This diversity is significant as it influences diagnostic testing, treatment regimens, and disease progression.
- Group N (New): This group is much rarer and mainly found in West and Central Africa, particularly in countries like Gabon and Cameroon. It is believed to have originated from a separate zoonotic transfer from chimpanzees, similar to Group M.
- Group O (Outlier): Like Group N, this group is also primarily confined to West and Central Africa but has been identified in other regions due to international travel. Group O represents another zoonotic transfer and is characterized by unique viral properties that distinguish it from the more common strains of HIV-1.
- HIV-1: This is the more prevalent and widely studied type, responsible for the majority of global HIV infections. It is further categorized into several groups:
- HIV-2: This type is less prevalent than HIV-1 and is mostly found in West Africa. It exhibits a lower level of genetic diversity compared to HIV-1. Several subtypes, labeled A through H, have been identified within HIV-2. These subtypes are important for understanding the epidemiology of HIV-2, although they are generally associated with fewer infections globally.
- Significance of Classification:
- The classification of HIV into distinct types and groups is essential for various public health strategies. The diversity within HIV-1 necessitates tailored approaches for diagnostic testing and treatment, as responses may differ among the various subtypes. For instance, specific antiretroviral therapies may be more effective against certain subtypes, highlighting the importance of genetic diversity in therapeutic decisions.
- Additionally, understanding the geographic distribution of different HIV types and groups aids in monitoring and controlling outbreaks, especially in areas where particular strains are more prevalent.
Morphology of HIV Virus
The morphology of the Human Immunodeficiency Virus (HIV) is critical for understanding its function and mechanism of infection. This spherical virus has distinctive structural features that enable it to evade the immune system and establish infection.
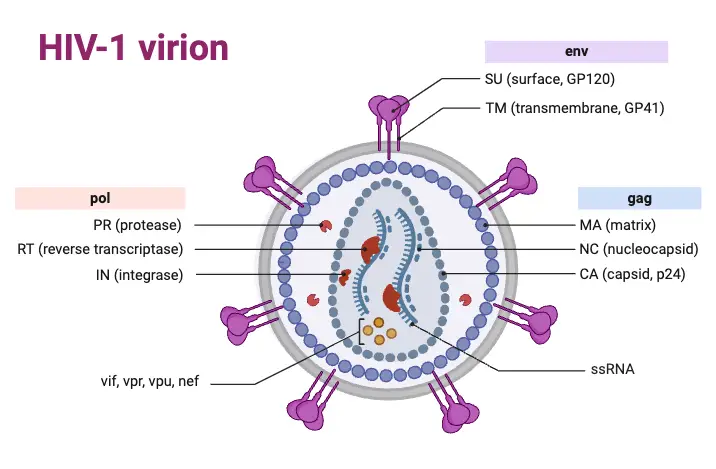
- General Structure:
- HIV measures approximately 120 nm in diameter and possesses a three-layered architecture. This structure includes:
- Innermost Genome Layer: Contains the viral RNA and associated proteins.
- Middle Nucleocapsid: This cone-shaped component encapsulates the viral genome.
- Outer Membrane: An envelope comprised of lipoproteins and glycoproteins, which plays a crucial role in viral entry into host cells.
- HIV measures approximately 120 nm in diameter and possesses a three-layered architecture. This structure includes:
- Viral Genome:
- The HIV genome is characterized as diploid, containing two identical copies of single-stranded positive-sense RNA. Its complexity sets it apart from other human retroviruses, comprising three primary genes:
- gag Gene: Encodes core proteins, specifically:
- p24: The major antigen detectable in the serum during the early stages of HIV infection.
- p15 and p18: Additional core proteins that contribute to the viral structure.
- pol Gene: This gene encodes essential enzymes, including:
- Reverse Transcriptase: Converts RNA into DNA, crucial for integrating the viral genome into host DNA.
- Integrase: Facilitates the incorporation of viral DNA into the host’s genome.
- Protease: Cleaves viral precursor proteins into functional components.
- env Gene: Codes for the precursor glycoprotein gp160, which is processed into:
- gp120: An outer envelope glycoprotein that forms spikes on the viral surface.
- gp41: A transmembrane protein that assists in membrane fusion.
- gag Gene: Encodes core proteins, specifically:
- The HIV genome is characterized as diploid, containing two identical copies of single-stranded positive-sense RNA. Its complexity sets it apart from other human retroviruses, comprising three primary genes:
- Regulatory Genes:
- In addition to the primary structural genes, HIV possesses several regulatory genes that are essential for its replication and pathogenicity. These include:
- tat Gene: Encodes the Tat protein, which enhances viral gene transcription.
- rev Gene: Controls the transport of viral mRNA from the nucleus to the cytoplasm.
- nef Gene: Suppresses the synthesis of major histocompatibility complex (MHC) proteins, which reduces the ability of cytotoxic T cells to target HIV-infected cells.
- Other regulatory genes include vif, vpr, vpu (in HIV-1), and vpx (in HIV-2).
- In addition to the primary structural genes, HIV possesses several regulatory genes that are essential for its replication and pathogenicity. These include:
- Nucleocapsid Structure:
- The nucleocapsid, which encases the viral genome, comprises proteins and houses three vital enzymes:
- Reverse Transcriptase: Functions both in synthesizing viral DNA and in degrading the viral RNA during replication.
- Integrase: Assists in integrating the proviral DNA into the host genome.
- Protease: Responsible for processing viral polyproteins into functional proteins.
- The nucleocapsid, which encases the viral genome, comprises proteins and houses three vital enzymes:
- Viral Envelope:
- HIV’s lipoprotein envelope is derived from the host cell membrane and incorporates virus-coded glycoproteins. The outer spikes, primarily composed of gp120, engage with CD4 receptors on potential host cells, facilitating viral entry. The transmembrane glycoprotein gp41 aids in the fusion of the virus with the host cell membrane.
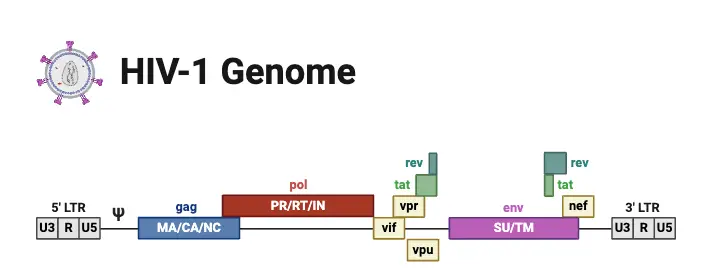
Viral Genes and Antigens
Understanding the viral genes and antigens of HIV is essential for grasping its mechanisms of infection and pathogenesis. The HIV genome is characterized by various structural and regulatory genes that contribute to the virus’s ability to replicate and evade the host’s immune system.
- Structural Genes:
- The genome of HIV comprises three main structural genes:
- gag: This gene encodes proteins that form the virus’s internal structure. Key products include:
- p24: The principal core antigen, detectable in the serum during early infection.
- p15 and p18: Additional proteins that also contribute to the nucleocapsid structure.
- pol: Encodes essential enzymes for viral replication, including:
- Reverse Transcriptase: Converts the viral RNA genome into DNA.
- Integrase: Integrates the viral DNA into the host cell’s genome.
- Protease: Cleaves precursor polyproteins into active viral proteins.
- env: This gene codes for envelope proteins, specifically:
- gp160: A precursor that is cleaved into gp120 and gp41, which are critical for viral entry into host cells.
- gag: This gene encodes proteins that form the virus’s internal structure. Key products include:
- The genome of HIV comprises three main structural genes:
- Regulatory Genes:
- Besides the structural genes, HIV also possesses several regulatory genes crucial for its replication:
- tat: Encodes the Tat protein, enhancing viral gene transcription.
- rev: Controls the transport of viral mRNA from the nucleus to the cytoplasm.
- nef: Inhibits MHC class I synthesis, reducing the immune system’s ability to recognize and kill infected cells.
- Other regulatory genes include vif, vpr, and the unique vpu in HIV-1, which contribute to various aspects of viral replication and immune evasion.
- Besides the structural genes, HIV also possesses several regulatory genes crucial for its replication:
- Antigenic Variability:
- HIV exhibits significant antigenic variability, particularly between the two main types, HIV-1 and HIV-2. While both types share some core polypeptides, their envelope antigens differ markedly. HIV-2 is less virulent and primarily confined to West Africa, showing only 40% genetic identity with HIV-1.
- HIV-1 is further divided into at least ten subtypes (designated A to J) based on genetic sequencing, with Group M (major) encompassing the majority of global infections. Geographic distribution of these subtypes varies, influencing their transmissibility through different routes.
- Serodiagnostic Implications:
- The antigenic differences between various HIV strains are vital for diagnostic testing. For example, infections by atypical strains (like Group O) may evade detection if the diagnostic tests do not incorporate the relevant antigens. Therefore, understanding these differences can significantly impact serodiagnosis.
- Viral Isolation:
- HIV can be isolated from various bodily fluids, including blood, semen, cervical secretions, saliva, and breast milk. This versatility underscores the importance of understanding both the genes and antigens in relation to transmission dynamics.
- Immunological Effects:
- The primary pathogenic mechanism of HIV involves the depletion of CD4+ T lymphocytes, leading to a reversal of the T helper (CD4+) to T suppressor (CD8+) cell ratio. This depletion results in impaired immune responses, making individuals susceptible to opportunistic infections.
- Interestingly, the immune dysregulation caused by HIV includes polyclonal activation of B lymphocytes, leading to hypergammaglobulinemia. This phenomenon results in elevated immunoglobulin levels, particularly IgG and IgA, which may not be effective against HIV but contribute to a range of immune-related complications.
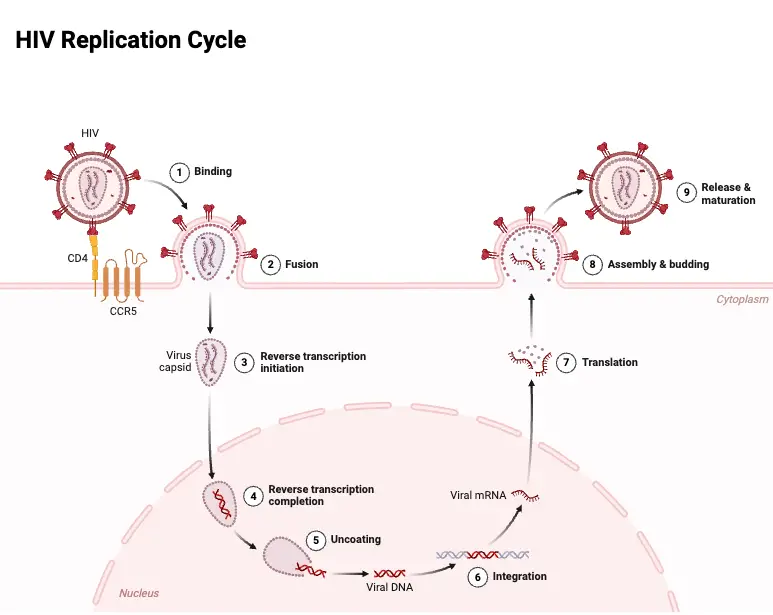
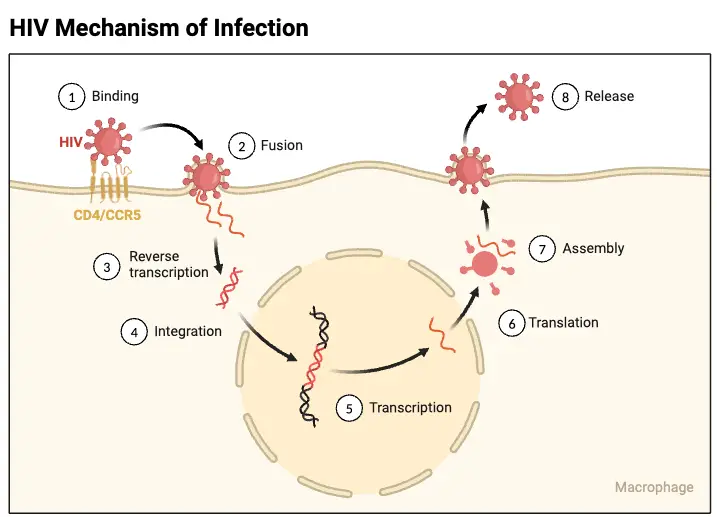
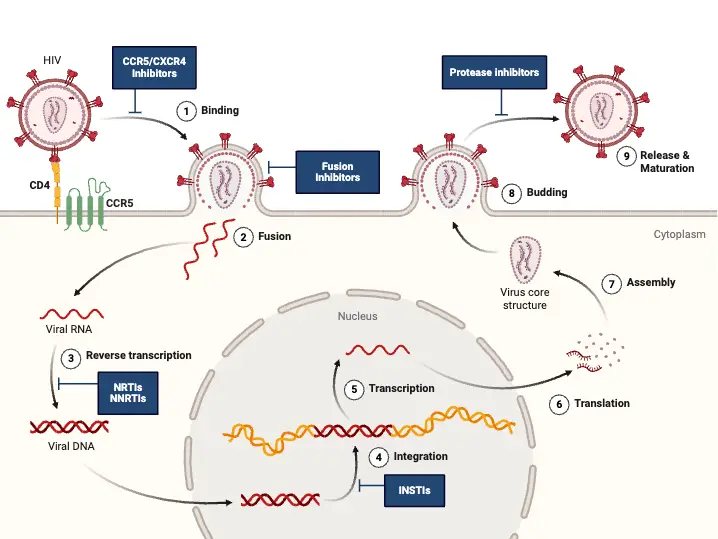
Viral Replication of HIV
The replication process of HIV is a complex and highly orchestrated series of events that enable the virus to infect host cells and propagate within the body. As a retrovirus, HIV employs its unique genetic machinery to reverse transcribe its RNA into DNA, subsequently integrating into the host genome and hijacking the host’s cellular mechanisms for its replication.
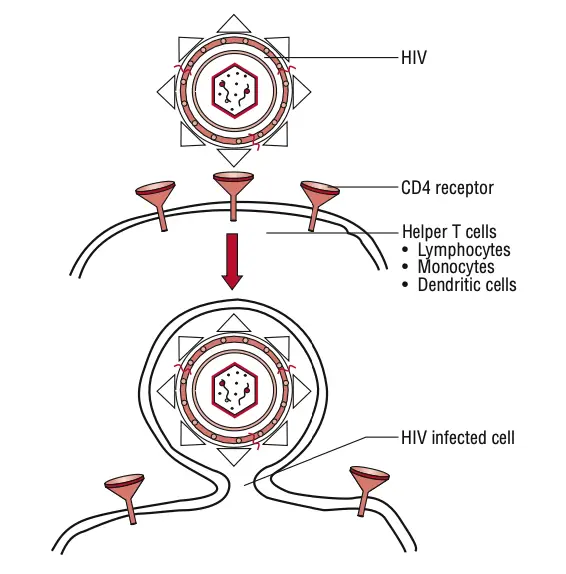
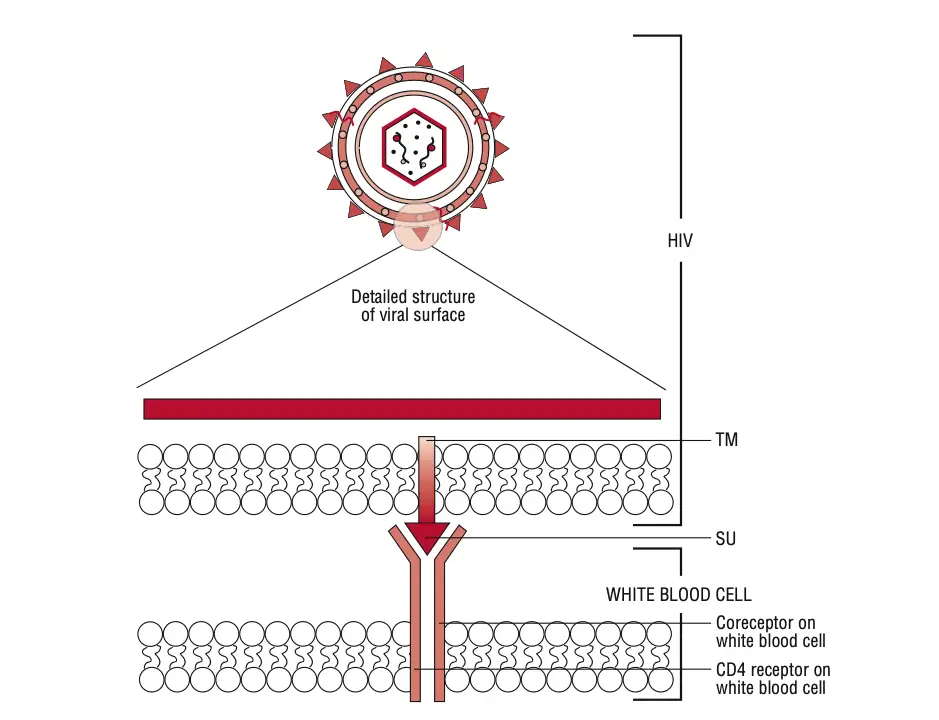
- Binding and Entry:
- HIV begins its replication cycle by binding to CD4 receptors on the surface of host cells, primarily targeting CD4+ T lymphocytes. This binding is facilitated by the viral gp120 envelope protein.
- The interaction between gp120 and CD4 is essential but insufficient for entry. Therefore, gp120 also engages with specific chemokine receptors, namely CXCR4 and CCR5.
- CXCR4 is primarily used by T-cell-tropic strains of HIV, while CCR5 is targeted by macrophage-tropic strains.
- Once the binding occurs, the gp41 protein of the virus mediates the fusion of the viral envelope with the host cell membrane, allowing the viral contents to enter the cell.
- Uncoating and Reverse Transcription:
- Following entry, the virus undergoes uncoating, where the viral capsid is disassembled, releasing the viral RNA into the host cell’s cytoplasm.
- The enzyme RNA-dependent DNA polymerase, also known as reverse transcriptase, synthesizes double-stranded DNA from the viral RNA genome. This step is crucial, as the newly formed viral DNA must integrate into the host cell’s genome.
- Integration:
- The integration of viral DNA into the host genome is mediated by the viral enzyme integrase. This integration creates a provirus, which is essentially the HIV genome incorporated into the host DNA.
- Transcription and Translation:
- Once integrated, the host cell’s RNA polymerase transcribes the proviral DNA into viral mRNA. This mRNA serves as a template for the synthesis of various viral proteins.
- The viral mRNA is translated to produce precursor polyproteins. These include:
- Gag: Cleaved by the viral-encoded protease into several proteins, including the main core protein (p24) and matrix protein (p17).
- Pol: This polyprotein is also processed by protease to yield essential enzymes such as reverse transcriptase, integrase, and protease itself.
- Env: This protein is cleaved by a cellular protease into components essential for forming the viral envelope.
- Assembly and Budding:
- The assembly of immature virions occurs in the cytoplasm, where the precursor polyproteins gather at the cell membrane.
- As the immature virion buds off from the host cell, the viral protease cleaves the precursor polyproteins. This cleavage is a critical step, as it converts the immature virion into a mature, infectious HIV particle.
Antigenic and Genomic Properties of HIV
The human immunodeficiency virus (HIV) is characterized by distinct antigenic and genomic properties that play critical roles in its pathogenicity and the immune response it elicits. Understanding these features is essential for developing diagnostic tools, treatments, and potential vaccines.
- Antigenic Properties:
- HIV presents two primary types of antigens: group-specific antigens and type-specific envelope glycoproteins.
- The group-specific antigen, represented by the protein p24, is found in the viral core. This protein remains stable, displaying no significant variations. While serum antibodies against p24 serve as important serological markers for HIV diagnosis, they do not neutralize the virus’s infectivity or provide protective immunity.
- Type-specific envelope glycoproteins, specifically gp120 and gp41, are located on the viral surface.
- gp120 protrudes from the virus and is crucial for binding to CD4 receptors and chemokine receptors on CD4+ T cells. It exhibits considerable antigenic variation due to mutations in its encoding gene. The V3 loop of gp120 is notably immunogenic and also shows significant antigenic diversity.
- Antibodies targeting gp120 can neutralize viral infectivity; however, the rapid emergence of gp120 variants complicates the development of effective vaccines, as these variants may evade the immune response.
- gp41, which is embedded in the viral envelope, is essential for mediating the fusion of the viral envelope with the host cell membrane during infection.
- Genomic Properties:
- HIV is categorized into two distinct types: HIV-1 and HIV-2. Both types possess unique envelope antigens; however, their core polypeptides demonstrate some degree of cross-reactivity.
- HIV-1 is the predominant strain found in the Americas, Europe, and other Western countries, while HIV-2 primarily originates from Western Africa and is more closely related to the simian immunodeficiency virus (SIV) than HIV-1.
- Based on sequence analysis of the gag or env genes, HIV-1 and HIV-2 strains are classified into three groups:
- Group M (major or main), which is the most prevalent and responsible for the majority of global HIV-1 infections, consists of nine subtypes: A, B, C, D, F, G, H, J, and K. These subtypes primarily originated from Central Africa but are now widespread globally.
- Group N (non-M, non-O/new) and Group O (other) encompass only a few HIV-1 isolates from Central Africa, distinct from Group M.
Pathogenesis of HIV
The pathogenesis of HIV infection involves a series of complex interactions between the virus and the host’s immune system. This process initiates in the genital tract and subsequently affects various cellular components, leading to significant immunological consequences.
- Initial Infection:
- Infection with HIV begins in Langerhans cells, which are specialized dendritic cells located in the mucosal surfaces of the genital tract.
- The virus subsequently infects local CD4+ T helper cells, leading to the appearance of HIV in the bloodstream typically within 4 to 11 days post-exposure.
- Cellular Targets:
- CD4 receptors are present on a range of immune cells, including CD4 T lymphocytes, monocytes, macrophages, and dendritic cells. This widespread expression allows for the efficient spread of HIV throughout the body.
- The gp120 protein is crucial for HIV’s pathogenicity, determining the virus’s cellular tropism. The V3 region of gp120 is particularly important in this regard, as it dictates which specific CD4 cell types the virus will infect.
- Mechanism of Infection:
- Upon entry into the host, gp120 selectively binds to CD4 surface receptors and either CCR5 or CXCR4 chemokine receptors found on macrophages.
- Following this binding, the associated gp41 protein facilitates the fusion of the viral envelope with the host cell membrane.
- This fusion results in the release of the viral RNA and associated proteins into the cytoplasm, where reverse transcription converts the RNA genome into double-stranded DNA, forming a provirus.
- Latent Infection:
- The integrated provirus becomes part of the host cell’s genome, leading to a latent infection. This latency can contribute to a prolonged incubation period during which high levels of viral replication occur, estimated at 10 billion HIV particles produced and destroyed daily.
- During this latent phase, gp120 on the surface of infected cells can lead to the formation of multinucleated syncytia, where infected cells fuse with uninfected cells. This fusion can ultimately result in the lysis of the fused cells, causing further depletion of CD4+ T cells.
- Cytopathic Effects:
- HIV induces several cytopathic effects, including the accumulation of nonintegrated circular DNA and increased permeability of the plasma membrane, which can lead to apoptosis of infected T cells.
- The release of progeny virions from lysed cells facilitates the infection of new cells, thereby perpetuating the cycle of infection. As the virus spreads, the number of CD4+ T cells declines significantly, reversing the CD4cell ratio and impairing the overall immune function.
- Viral Persistence in Lymph Nodes:
- Continuous replication occurs in the lymph nodes, where the virus disseminates virions and infected T cells into the bloodstream.
- Over the course of infection, a dramatic reduction in CD4+ T cell numbers is observed. This decline can occur due to various mechanisms, including HIV-induced cytolysis, immune-mediated cytotoxicity, and the natural differentiation processes of T cells.
- Central Nervous System Involvement:
- HIV also targets brain monocytes, resulting in the formation of multinucleated giant cells and significant central nervous system manifestations. The fusion of HIV-infected cells within the brain and other tissues represents a key pathological finding in HIV infection.
HIV Virus Genome and Proteins
The HIV virus, a member of the Retroviridae family, possesses a unique genome structure and a sophisticated mechanism for protein production. Understanding these components is vital for comprehending how the virus replicates and interacts with host cells.
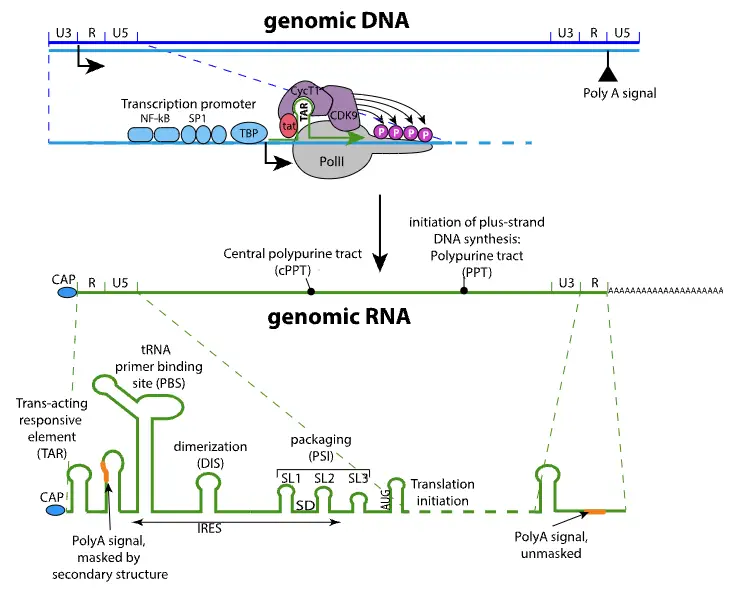
- Genome Structure:
- HIV features a monopartite, linear, dimeric single-stranded RNA (+) genome, measuring approximately 9.75 kilobases in length.
- At both the 5′ and 3′ ends of the genome, there are two long terminal repeats (LTRs), each around 600 nucleotides long. These LTRs play critical roles in transcription and integration into the host genome.
- The genome includes important elements such as a 5′-cap and a 3′-poly-A tail, which are essential for mRNA stability and translation efficiency.
- Additionally, the 5′ end of the genome contains a primer binding site (PBS), while the 3′ end features a polypurine tract (PPT), both crucial for reverse transcription.
- Transcription and Splicing:
- The transcription of the HIV genome is initiated by promoter elements located in the 5′ LTR, which interact with cellular DNA-directed RNA polymerase.
- Initially, the first polyA site located within the R region is not recognized due to secondary RNA structures. However, the presence of the U3 sequence upstream of R resolves this issue, allowing proper recognition of the polyA site and the formation of complete viral mRNA.
- Protein Production:
- HIV mRNA undergoes splicing, resulting in different types of mRNAs that encode various proteins essential for the virus’s lifecycle. Fully spliced mRNAs are responsible for coding the accessory proteins Rev, Tat, and Nef.
- Cellular mechanisms typically restrict unspliced RNA from exiting the nucleus. However, the viral protein Rev facilitates the export of incompletely or unspliced mRNA from the nucleus to the cytoplasm. This step is crucial for the virus’s replication process.
- Incompletely spliced mRNAs encode the envelope proteins, including SU (surface protein) and TM (transmembrane protein), as well as the accessory proteins Vif, Vpu, and Vpr. The unspliced full-length mRNA serves dual purposes: it acts as the genomic RNA packaged into new virions and serves as a template for translating the Gag and Gag-Pro-Pol polyproteins. Notably, this translation process involves a ribosomal frameshift, which is a unique feature of HIV’s protein synthesis.
Host Immunity Against Pathogenesis of HIV Infection
The immune response to HIV infection involves both cell-mediated and humoral components. Understanding these mechanisms is crucial for comprehending how HIV undermines the immune system, leading to its pathogenesis.
- Cell-Mediated Immunity (CMI):
- CMI involves the activation of various immune cells that respond to HIV proteins. CD4+ T helper cells, monocytes, and macrophages are pivotal in this response.
- CD4+ T helper cells are particularly significant as they facilitate the activation of other immune cells, including cytotoxic T cells, natural killer (NK) cells, and B cells. This activation is essential for mounting an effective immune response against the virus.
- However, HIV binds directly to the CD4 receptors on T helper cells, resulting in their gradual depletion. This loss profoundly impacts both the cellular and humoral immune responses.
- A deficiency in CD4+ T cells leads to:
- Depression of the cellular immune response.
- Impairment of humoral responses, including the development of antibodies.
- Inability to mount delayed-type hypersensitivity reactions, which are crucial for fighting off opportunistic infections.
- Role of Monocytes and Macrophages:
- Monocytes and macrophages are also critical in HIV pathogenesis. They express CCR5 chemokine receptors, which serve as major coreceptors for HIV entry. These cells are often the primary targets of HIV in the brain, contributing to neurological complications associated with the infection.
- Infected pulmonary alveolar macrophages may participate in the development of interstitial pneumonitis, a condition seen in some HIV patients.
- Early in infection, macrophage-tropic strains of HIV predominate, facilitating transmission. The virus can infiltrate the brain via infected monocytes, releasing neurotoxic factors and chemotactic agents that attract inflammatory cells.
- Humoral Immunity:
- Humoral immunity is characterized by the production of neutralizing antibodies against HIV proteins such as p24, gp120, and gp41. Despite the presence of these antibodies, the levels of neutralizing activity are often low.
- In adults, antibodies against gp120 typically develop several months after initial viremia. The presence of these neutralizing antibodies is generally associated with a slower progression of disease in individuals across all age groups.
- Mechanisms of HIV Evasion:
- HIV employs several strategies to evade the host’s immune defenses:
- Inactivation of Key Immune Defenses: HIV infects lymphocytes and macrophages, diminishing the activation of the immune system and contributing to delayed-type hypersensitivity.
- Evasion of Antibody Detection: The virus utilizes antigenic drift of the gp120 protein, making it more difficult for the immune system to recognize and mount a response.
- Heavy Glycosylation of gp120: This modification masks the viral antigens from the immune system, further aiding in evasion.
- HIV employs several strategies to evade the host’s immune defenses:
HIV Life Cycle and Host Immune Responses
The life cycle of HIV is intricate, involving a series of steps that enable the virus to infect host cells, replicate, and evade the immune system. Understanding this cycle and the corresponding immune responses provides insight into the challenges of combating HIV infection.
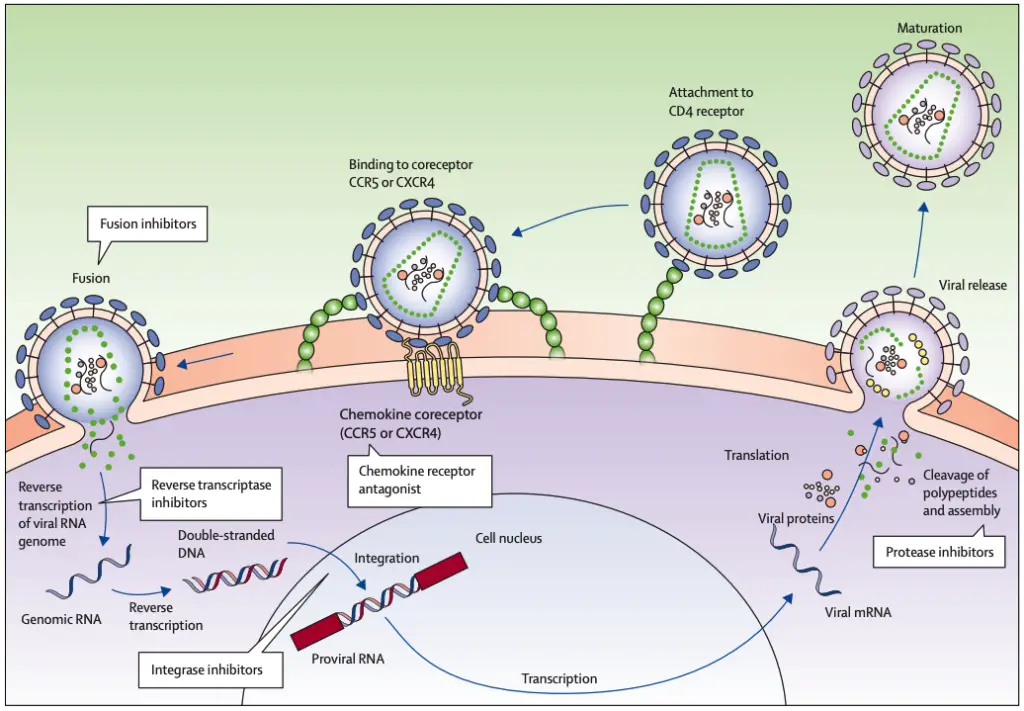
- Target Cells:
- The primary targets of HIV are activated CD4 T lymphocytes. The virus gains entry through interactions with the CD4 receptor and chemokine coreceptors, specifically CCR5 or CXCR4.
- In addition to activated CD4 T cells, other immune cells such as resting CD4 T cells, monocytes, macrophages, and dendritic cells are also susceptible to infection.
- Notably, HIV can infect CD4-independent cells, including astrocytes and renal epithelial cells, where the expression of HIV genes contributes to pathologies such as HIV-associated neurocognitive disorder and nephropathy.
- Transmission and Initial Immune Response:
- HIV transmission typically occurs across mucosal membranes through a single founder virus. This virus possesses distinct phenotypic traits, including a preference for CCR5 usage, enhanced interactions with dendritic cells, and resistance to interferon-alpha.
- Following transmission, there is a rapid increase in viral replication accompanied by a substantial induction of inflammatory cytokines and chemokines. This initial immune response is notably more pronounced than the responses observed in other chronic viral infections, such as hepatitis B or C.
- Viral Load Dynamics:
- After the initial spike in viral load, a decrease occurs, reaching a setpoint established by the host’s innate and adaptive immune responses. This setpoint is critical as it influences the long-term progression of the infection.
- HIV-specific CD8 T cells actively kill productively infected cells shortly after infection. However, the virus’s adaptive immune response leads to the emergence of mutations in key epitopes, facilitating immune escape.
- Role of CD8 T Cells and Exhaustion:
- In some individuals, particularly those with certain HLA types like HLA-B27, effective immune responses can develop. These responses are characterized by HIV-specific T cells that exhibit high avidity and polyfunctionality.
- Despite initial successes, nearly all individuals experience progressive exhaustion of HIV-specific T cells. This exhaustion is marked by high levels of programmed death-1 (PD-1) expression, leading to diminished effector functions.
- Neutralizing Antibodies:
- Neutralizing antibodies typically begin to appear about three months post-transmission. However, their emergence often results in the selection of viral escape mutants.
- A minority of patients—approximately 20%—develop broadly neutralizing antibodies capable of targeting multiple HIV-1 subtypes. These antibodies arise from a high frequency of somatic mutations, which can take years to develop.
- Despite their potential, broadly neutralizing antibodies often fail to confer significant benefit to patients due to the continual development of escape mutants. Consequently, research is focused on creating new immunogens to enhance the production of these antibodies for vaccine development.
- Innate Immune Response:
- The innate immune response plays a crucial role in controlling HIV infection, primarily through the action of natural killer (NK) cells.
- Similar to the adaptive response, viral escape mutants emerge, which can limit the antiviral effects of NK cells.
Immune Dysfunction Due to HIV Infection
HIV infection is characterized by a progressive decline in the immune system, primarily through the depletion of CD4 T cells. This immune dysfunction poses significant challenges to the body’s ability to combat infections and diseases.
- CD4 T Cell Depletion:
- The hallmark of HIV infection is the progressive loss of CD4 T cells, which occurs due to both reduced production and increased destruction.
- In the early stages of infection, there is a transient reduction in circulating CD4 T cells, followed by a recovery to near-normal levels. However, this recovery is short-lived as CD4 T cell counts gradually decrease over time, typically by about 50 to 100 cells per microliter.
- The reduction in CD4 T cells is attributed to direct viral infection and bystander effects, such as the formation of syncytia and immune activation, leading to T cell proliferation and subsequent senescence.
- Impact on T-cell Homeostasis:
- A significant effect on T-cell homeostasis occurs very early in the gastrointestinal tract, where a dramatic loss of activated CD4 T cells is observed. This loss results in minimal recovery even after antiretroviral therapy.
- Alongside the overall depletion of CD4 T cells, there are specific changes in T-cell subsets. Notably, there is a preferential loss of T-helper 17 (Th17) cells and mucosal-associated invariant T (MAIT) cells, both of which play crucial roles in defending against bacterial infections.
- Consequences of Lymphoid Cell Depletion:
- The profound depletion of lymphoid cells within the gastrointestinal tract leads to significant gastrointestinal dysfunction. This includes apoptosis of enterocytes and increased permeability of the gastrointestinal lining, which facilitates the entry of microbial products into the bloodstream.
- Elevated plasma concentrations of microbial products, such as lipopolysaccharides, are a direct consequence of this dysfunction and contribute to systemic inflammation, further exacerbating immune dysfunction.
- Destruction of Lymphoid Tissue Architecture:
- The destruction of the fibroblastic reticular cell network within lymphoid tissue leads to additional immune deficits. This architectural damage restricts access to interleukin-7 (IL-7), a vital T-cell survival factor, thereby impacting the replenishment of both CD4-naive and CD8-naive T cells.
- The overall impact of these processes results in a compromised immune system, increasing vulnerability to opportunistic infections and malignancies.
Immune Activation in HIV Infection
HIV infection is marked by significant immune activation, affecting both the adaptive and innate immune systems, as well as causing abnormalities in coagulation. This immune activation contributes to a complex interplay of factors that influence disease progression and patient outcomes.
- Drivers of Immune Activation:
- The immune activation in HIV-infected individuals is largely driven by several mechanisms:
- Direct Effects of HIV: The virus acts as a ligand for Toll-like receptors (TLR7 and TLR8) present on plasmacytoid dendritic cells. This interaction leads to the production of interferon-alpha, an important cytokine in the immune response.
- Microbial Translocation: The presence of microbial products, particularly lipopolysaccharides, activates TLR4, resulting in the production of pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha (TNFα). This translocation occurs when the gut barrier is compromised, allowing bacteria to enter the bloodstream.
- Co-infections: Co-infection with other viruses, notably cytomegalovirus (CMV), can induce a marked expansion of CMV-specific T cells, further driving immune activation.
- Altered T-Cell Ratios: There is often a reduced ratio of T-helper-17 (Th17) cells to regulatory T cells, particularly in the gastrointestinal tract, which plays a crucial role in maintaining mucosal immunity.
- The immune activation in HIV-infected individuals is largely driven by several mechanisms:
- Residual Inflammation:
- Even among individuals receiving antiretroviral therapy (ART) who achieve adequate CD4 T-cell restoration, evidence of residual inflammation persists.
- This residual inflammation has been associated with various health complications, including increased mortality, cardiovascular disease, cancer, neurological conditions, and liver disease.
- The markers of this persistent inflammation, such as elevated cytokine levels, indicate ongoing immune activation despite effective viral suppression.
- Impact of Antiretroviral Therapy:
- The addition of integrase inhibitors, like raltegravir, to ART regimens has shown to reduce T-cell activation in approximately one-third of participants with virological suppression.
- These findings suggest that low-level HIV replication may play a role in sustaining inflammation, even in well-controlled cases.
- Management of Co-infections:
- Addressing co-infections, such as cytomegalovirus and hepatitis C, has been shown to further decrease T-cell activation. This highlights the importance of comprehensive care strategies in managing HIV-related immune dysfunction.
- Potential Therapies and Future Directions:
- While many studies have established associations between inflammatory biomarkers and adverse clinical outcomes, determining causation has been challenging. Current strategies aimed at reducing residual inflammation in HIV-infected individuals primarily rely on small observational studies.
- Various existing medications, including statins, aspirin, angiotensin-converting enzyme inhibitors, and hydroxychloroquine, have shown potential to mitigate HIV-associated inflammation. However, randomized controlled trials are needed to ascertain whether targeting inflammation in virologically suppressed individuals on ART will yield clinically significant benefits.
Clinical Syndromes
The progression of untreated HIV infection typically spans over a decade, advancing through several distinct stages: primary infection, dissemination of the virus, clinical latency, and ultimately, a late stage known as AIDS. Each stage is characterized by specific clinical syndromes, reflecting the varying impacts of HIV on the immune system and overall health.
- Acute HIV Infection:
- This initial phase occurs approximately 3 to 6 weeks after exposure, marked by a rapid increase in plasma viral load and a corresponding drop in CD4 T cell count.
- Patients often experience nonspecific symptoms such as low-grade fever, fatigue, malaise, rash, headache, and lymphadenopathy. These symptoms may spontaneously resolve within weeks.
- Notably, during this period, serum antibodies are typically absent, although the p24 antigen can be detected, indicating the onset of what is termed seroconversion illness.
- Asymptomatic HIV Infection:
- Following acute infection, patients may enter an asymptomatic or clinically latent stage that can last several months to years. During this time, there is low-level viral replication, and CD4 counts gradually decline.
- Serological tests reveal the presence of HIV antibodies, while patients may exhibit persistent generalized lymphadenopathy. This condition is defined by the presence of enlarged lymph nodes in two or more noncontiguous extrainguinal sites lasting at least three months without any other illness that could cause such swelling.
- Although generally benign, this stage may eventually progress to AIDS-related complex (ARC) or AIDS.
- AIDS-Related Complex (ARC):
- ARC is characterized by symptoms such as lymphadenopathy and fever, often accompanied by malaise and weight loss. Patients may also experience diarrhea, night sweats, and fatigue.
- This syndrome can develop insidiously and may indicate impending progression to full-blown AIDS, typically within a few months.
- AIDS:
- AIDS represents the final and most severe stage of HIV infection, denoting significant immunosuppression and heightened vulnerability to a range of opportunistic infections and unusual malignancies, such as Kaposi’s sarcoma.
- The diagnosis is associated with a profound decline in CD4 T cells, falling below 200 cells/μL, along with increased viral loads and the presence of p24 antigen in the blood.
- Clinical manifestations of AIDS vary widely and can include:
- HIV Wasting Syndrome: Characterized by unintentional weight loss and diarrhea lasting over a month.
- Opportunistic Infections: Severe infections caused by pathogens that rarely affect immunocompetent individuals. Common infections include:
- Protozoal: Toxoplasmosis, cryptosporidiosis, and isosporiasis.
- Bacterial: Extrapulmonary tuberculosis, Mycobacterium avium-intracellulare complex, and various pyogenic infections.
- Viral: Cytomegalovirus disease, herpes simplex infections, and progressive multifocal leukoencephalopathy (PML).
- Fungal: Pneumocystis jirovecii pneumonia, candidiasis, cryptococcosis, and histoplasmosis.
- Malignancies: Patients with AIDS are particularly susceptible to certain cancers, notably:
- Kaposi’s Sarcoma: A vascular tumor significantly more prevalent in untreated AIDS patients.
- Lymphomas: Both Hodgkin’s and non-Hodgkin’s types are commonly observed, as well as cervical and anogenital cancers.
- Neurological Complications:
- Various neurological syndromes are associated with AIDS, including AIDS dementia complex, which arises from HIV’s infection of microglial cells and neurons, leading to cognitive deficits, memory loss, and behavioral changes.
- Other common conditions include subacute encephalitis and peripheral neuropathy, along with opportunistic infections affecting the central nervous system, such as toxoplasmosis and cryptococcal meningitis.
- Pediatric AIDS:
- Pediatric AIDS often results from vertical transmission from infected mothers, either during pregnancy or breastfeeding. Clinical manifestations typically emerge by age two, leading to a poor prognosis.
- Children may exhibit severe conditions such as pneumonia, oral candidiasis, lymphadenopathy, and growth retardation. The disease can progress rapidly, particularly in neonates whose immune systems are not fully developed.
- Interestingly, the progression of vertically acquired HIV in children can be categorized into three patterns: rapid progression, chronic progression, or adult-like infection patterns. Without treatment, the prognosis remains grim.
Geographical Distribution of HIV
HIV infection presents a significant global public health challenge, with varying geographical distribution patterns reflecting the diverse nature of the virus and its transmission dynamics. The two primary strains of HIV, HIV-1 and HIV-2, have distinct epidemiological profiles and prevalence rates across different regions.
- HIV-1 Variants:
- The most prevalent strain globally is HIV-1, with specific subtypes showing marked geographical preferences:
- Subtype A predominantly occurs in West Africa.
- Subtype B is primarily found in the United States, Europe, and Australia.
- Subtype C is prevalent in India, China, and Southern Africa.
- The most prevalent strain globally is HIV-1, with specific subtypes showing marked geographical preferences:
- HIV-2 Presence:
- While less common, HIV-2 is a significant contributor to HIV epidemics, particularly in West Africa. It is also observed in several European countries, indicating a varied transmission landscape.
- Global Prevalence Statistics:
- Since the initial recognition of AIDS in the United States in 1981, HIV has evolved into a worldwide epidemic, with an estimated 38 million individuals living with the virus globally.
- The World Health Organization (WHO) estimates that there are 5 million new HIV infections each year, with 90% of these cases occurring in developing nations, particularly in sub-Saharan Africa and Southeast Asia.
- Impact on Pregnant Women:
- The HIV seroprevalence rate among pregnant women is notably high in sub-Saharan Africa, ranging between 35% and 45%. In contrast, Asia reports a significantly lower rate of 2%.
- The vertical transmission rate of HIV from infected mothers to infants is approximately 24% without breastfeeding. In India, this transmission rate can escalate to 48% among breastfeeding mothers.
- Children and HIV:
- The epidemic has severely impacted children, with over 4.4 million infected worldwide and approximately 3.2 million deaths attributed to HIV infection among this vulnerable population.
- HIV in India:
- India first documented HIV infections among sex workers in Chennai, Madurai, and Vellore during 1986–1987. The first AIDS case in the country was also reported in Mumbai in 1986.
- By 2008, the National AIDS Control Organisation (NACO) estimated around 2.4 million individuals living with HIV in India, making it one of the countries with the highest prevalence, second only to South Africa.
- The national prevalence of HIV in India varies between 0.4% and 1.3%, with distinct differences observed across various states classified as high-, medium-, and low-prevalence areas.
- State-Specific Data:
- States like Tamil Nadu, Andhra Pradesh, Karnataka, Maharashtra, and Goa are categorized as high-prevalence areas, exhibiting rates over 1% even within low-risk populations.
- Both HIV-1 and HIV-2 strains are found in India, with subtype C showing high prevalence. Specifically, studies indicate that 78.4% of strains in North India and 95% of strains in Kolkata and South India are identified as HIV-1 subtype C.
Reservoir, Source, and Transmission of HIV Infection
Understanding the reservoir, source, and transmission mechanisms of HIV is crucial for addressing its spread and mitigating its impact on global health. HIV primarily infects humans, where infected individuals serve as the main reservoir of the virus. The pathways of transmission reveal how the virus propagates among different populations.
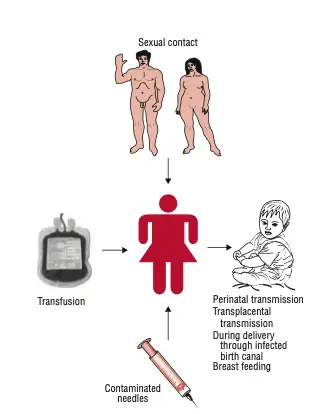
- Reservoir of HIV:
- Humans infected with HIV are the primary reservoir.
- High concentrations of HIV are found in bodily fluids such as blood, semen, and vaginal secretions.
- Additionally, the virus can be present in the breast milk of an infected mother, further facilitating potential transmission.
- Sources of Infection:
- The principal sources of HIV infection include:
- Blood from HIV-positive individuals.
- Semen and vaginal secretions during sexual contact.
- Breast milk from infected mothers.
- The principal sources of HIV infection include:
- Modes of Transmission:
- Sexual Transmission:
- This method accounts for more than 70% of all HIV infections globally.
- It occurs more frequently among heterosexual individuals than among homosexual men.
- Specific behaviors increase the likelihood of transmission:
- Having multiple sexual partners.
- Engaging in sex with commercial sex workers.
- Participating in receptive anal sex.
- The presence of other sexually transmitted infections (STIs), such as gonorrhea, syphilis, or herpes simplex virus type 2, can increase the risk of HIV transmission by over 100 times.
- Inflammation and ulcers from these STIs facilitate the virus’s entry through mucosal surfaces.
- Transmission through Blood Transfusion:
- HIV can be transmitted through transfusions of contaminated blood or blood products, including serum and plasma from infected individuals.
- Organ transplants from HIV-positive donors can also pose a risk.
- Though rare, health care workers may contract HIV through needle stick injuries involving infected blood, with a reported transmission risk of approximately 0.3%.
- Parenteral Transmission:
- This route is particularly significant among intravenous (IV) drug users, who often share contaminated needles.
- In regions like Manipur in India, the prevalence of HIV among injecting drug users has surged dramatically, rising from 2-3% in 1989 to over 75% by the early 2000s. This stark increase underscores the impact of shared injecting equipment.
- Mother-to-Child Transmission:
- Vertical transmission can occur during pregnancy, childbirth, or breastfeeding.
- In utero infection may happen if the fetus comes into contact with HIV via the placenta or through inflamed amniotic membranes. The risk of transmission increases with the duration of exposure to maternal blood and cervical secretions during delivery.
- Perinatal transmission may happen during the birthing process or through breastfeeding, with substantial rates observed in various studies.
- Approximately one-third to half of perinatal HIV infections in Africa are attributed to breastfeeding.
- In untreated women, the transmission rate to infants is 13% in Europe and 40% in Africa, highlighting geographical disparities.
- In India, the mother-to-infant transmission rate ranges from 36% to 40%, influenced by breastfeeding practices.
- Sexual Transmission:
- High-Risk Populations:
- Individuals at the highest risk for HIV infection include:
- Sexually active individuals, both heterosexual and homosexual.
- IV drug users and their sexual partners.
- Newborns of HIV-positive mothers.
- Individuals at the highest risk for HIV infection include:
Diagnostic Tests of HIV Infection
HIV diagnostic tests are essential for identifying individuals infected with the virus and guiding treatment and prevention strategies. Various testing methodologies have been developed over the years, each with distinct characteristics and applications. Here’s an overview of the primary diagnostic tests used for HIV infection.
- Health Canada Guidelines: In Canada, only tests approved by Health Canada are utilized by diagnostic laboratories to ensure accuracy and reliability in HIV testing.
- HIV Enzyme Immunoassay (EIA):
- The EIA is a widely employed screening tool for HIV and other infectious diseases due to its high sensitivity and automation capabilities, facilitating large-scale testing.
- Since the 1980s, HIV EIAs have improved in sensitivity and specificity, significantly reducing the ‘window period’—the time from exposure to detectable antibodies—from over 12 weeks to generally less than three weeks.
- Although highly sensitive, these tests can yield false-positive results, which are influenced by the assay type and the HIV prevalence in the tested population. Therefore, it is crucial for laboratories to confirm positive EIA results with additional testing, typically through Western blot assays.
- Some laboratories may use a second EIA that targets a different viral antigen to confirm results before proceeding with Western blot testing.
- p24 Antigen Testing:
- The p24 antigen test is another EIA-based method that detects the p24 antigen, which can sometimes appear before HIV antibodies in recently infected individuals.
- This test is particularly valuable for patients who are symptomatic but yield negative EIA results, or for those who are EIA-positive yet Western blot-negative or indeterminate.
- When p24 antigen is detected without corresponding antibodies, a follow-up antibody test is recommended, as seroconversion typically occurs within weeks following the initial screening. However, not all seroconverting patients will show detectable p24 antigen.
- Western Blot:
- The Western blot is an immunoblotting technique that identifies specific antibodies to various HIV proteins in patient serum.
- In this process, serum is exposed to a nitrocellulose strip that contains all HIV proteins arranged by molecular weight. If antibodies are present, they will bind to these proteins, leading to a visual banding pattern when reacted with a color development solution.
- To be considered positive, a specimen must show at least one core band and one envelope band. Indeterminate results, where bands are present but do not meet positivity criteria, necessitate follow-up testing within three to four weeks.
- If follow-up yields a similar banding pattern, the majority of patients are likely HIV-negative. In cases of indeterminate results, especially for patients with risk factors, qualitative PCR may be utilized for confirmation.
- Qualitative PCR:
- PCR, or polymerase chain reaction, amplifies viral nucleic acid, enabling detection even at low viral loads. This method is particularly useful for diagnosing HIV in infants born to infected mothers, as maternal antibodies can mask infection.
- It is vital to confirm that the PCR assay can effectively detect the mother’s HIV subtype, especially if non-B HIV strains are suspected.
- Additionally, PCR can clarify indeterminate Western blot results and test immunocompromised patients who may not develop a typical antibody response.
- Quantitative RNA PCR and Genotyping:
- Quantitative RNA PCR is employed to monitor HIV-positive individuals, particularly regarding their response to antiretroviral therapy and to assess when treatment should begin.
- Genotyping, which detects drug resistance mutations, informs clinicians about optimal antiretroviral combinations for patients. However, quantitative PCR is not suitable for initial HIV diagnosis due to potential false results.
- Point-of-Care (POC) Testing:
- POC testing refers to testing conducted in various locations, such as clinics or hospitals, and is gaining traction in some regions of Canada, particularly in sexually transmitted infection clinics.
- Guidelines for POC testing stress the importance of using Health Canada-approved devices and implementing comprehensive quality assurance programs at testing sites.
- POC tests are recommended for situations requiring immediate results, such as following needlestick injuries or in high-risk peripartum scenarios. However, all positive or ambiguous POC results must be confirmed through standard serological testing.
Treatment of HIV Infection
The treatment of HIV infection primarily involves the use of antiretroviral therapy (ART), which is crucial for inhibiting the replication of the virus and improving patient outcomes. The main goal of ART is to lower the viral load, thereby reducing morbidity and mortality associated with HIV infection. Antiretroviral drugs can be classified into several categories, each playing a specific role in the management of HIV.
- Antiretroviral Drug Classes:
- Nucleoside Analog Reverse Transcriptase Inhibitors (NRTIs):
- Examples include Zidovudine (AZT), Didanosine (ddI), Zalcitabine (ddc), Lamivudine (3TC), Stavudine (d4T), and Abacavir.
- NRTIs function by inhibiting the reverse transcriptase enzyme, which is vital for HIV replication. They achieve this by mimicking natural nucleosides, leading to chain termination during DNA synthesis.
- AZT is particularly recommended for individuals with a CD4 count below 500 cells/µL and for pregnant women to minimize the risk of vertical transmission to the fetus. However, monotherapy with AZT may lead to resistance and toxicity, particularly at high doses.
- Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs):
- This class includes Nevirapine, Delaviridine, and Efavirenz.
- NNRTIs work by binding to the reverse transcriptase enzyme, causing a change in its structure and thereby inhibiting the enzyme’s function. This action disrupts the maturation of the virion, further preventing the virus from effectively replicating.
- Protease Inhibitors (PIs):
- Notable examples are Ritonavir, Indinavir, Saquinavir, Nelfinavir, and Amprenavir.
- PIs inhibit the protease enzyme, which is essential for the maturation of HIV particles during the late stages of the viral life cycle. By blocking this process, protease inhibitors help to prevent the release of infectious virions.
- Nucleotide Reverse Transcriptase Inhibitors:
- Tenofovir is a prominent example. Similar to NRTIs, it inhibits reverse transcriptase but has unique properties that enhance its effectiveness against HIV.
- Fusion Inhibitors:
- Enfuvirtide is a representative drug in this category, functioning by preventing the virus from entering host cells.
- Nucleoside Analog Reverse Transcriptase Inhibitors (NRTIs):
- Laboratory Diagnosis of HIV:
- Diagnosis of HIV infection involves a series of laboratory tests, which serve various purposes.
- Serology Tests:
- The ELISA test is commonly used for initial screening. Rapid tests, such as dot blot assays and latex agglutination, also serve as effective screening methods.
- Confirmation tests include the Western blot and indirect immunofluorescence test.
- Antigen Detection:
- The p24 antigen test can detect the virus in its early stages of infection.
- Viral RNA Detection:
- RT-PCR is employed to measure the amount of viral RNA in the blood, providing insights into the viral load.
- Serology Tests:
- Diagnosis of HIV infection involves a series of laboratory tests, which serve various purposes.
- Challenges in HIV Treatment:
- Monotherapy with any single class of antiretroviral agents has been associated with suboptimal clinical outcomes. The primary concern is the rapid development of drug-resistant HIV variants, which can occur during monotherapy. Resistance not only complicates treatment regimens but also contributes to cross-resistance among related drugs, diminishing the effectiveness of subsequent therapies.
Prevention and Control of HIV Infection
Effective prevention and control of HIV infection are critical in reducing transmission rates and managing the public health impact of the virus. Several key strategies are employed, including health education, screening of blood and blood products, infection control measures, and ongoing vaccine development.
- Health Education:
- Education is fundamental in the fight against HIV/AIDS, particularly in the absence of a widely available vaccine. The focus of health education is to promote behavioral changes and lifestyles that minimize the risk of transmission.
- Key components include:
- Safe Sexual Practices: Encouraging the use of condoms during sexual intercourse is essential, as condoms significantly reduce the risk of transmitting HIV.
- Needle Safety: Individuals are advised against sharing needles or syringes, which can facilitate the spread of the virus.
- Vertical Transmission Awareness: Providing information to HIV-positive women about the risks associated with vertical transmission is crucial for preventing transmission to infants.
- Screening of Blood and Blood Products:
- Rigorous screening protocols for blood donors are essential to ensure the safety of blood and blood products.
- All potential blood donors should be tested for HIV-1 and HIV-2 antibodies using reliable screening tests, such as the ELISA.
- Individuals who test positive for HIV must refrain from donating blood, plasma, organs, tissues, or sperm.
- Regular screening has significantly decreased the transmission of HIV through contaminated blood transfusions.
- Infection Control:
- Infection control practices are vital for healthcare settings and other environments where exposure to blood and body fluids is possible.
- Universal precautions should be adopted, including:
- Protective Clothing: The use of gloves, masks, gowns, and other barriers helps prevent direct exposure to potentially infectious materials.
- Disinfection Protocols: Contaminated surfaces must be disinfected using agents like 10% household bleach, 70% ethanol, or 2% glutaraldehyde.
- Laundry Practices: Washing clothes in hot water with adequate detergents effectively kills HIV.
- Vaccine Development:
- While a safe and effective vaccine against HIV is not yet available, research continues to address several challenges inherent to the virus.
- An ideal HIV vaccine would need to:
- Prevent the acquisition of the virus during sexual intercourse.
- Block transmission from HIV-positive mothers to their infants.
- Inhibit the progression of the disease once infected.
- Scientific obstacles include:
- Antigenic Diversity: The virus’s ability to mutate rapidly complicates vaccine development, as the immune response must target various forms of the virus.
- Mucosal Transmission: Initial protection requires the production of secretory antibodies to prevent the virus from entering through mucosal surfaces.
- Latency and Integration: The virus can remain dormant within host cells, evading the immune system, and its integration into host chromosomes complicates eradication efforts.
- Viral Escape Mutants: The high error rate of reverse transcriptase leads to rapid mutations, resulting in viral strains that can escape immune detection.
Research into potential vaccines has involved using gp120 or its precursor gp160 as immunogens in clinical trials. Institutions like the National AIDS Research Institute (NARI) in Pune, India, are evaluating various approaches, including DNA vaccines and modified vaccinia Ankara (MVA). Phase I clinical trials for these vaccines are underway, demonstrating ongoing commitment to finding an effective preventive solution against HIV.
- Fearon M. The laboratory diagnosis of HIV infections. Can J Infect Dis Med Microbiol. 2005 Jan;16(1):26-30. doi: 10.1155/2005/515063. PMID: 18159524; PMCID: PMC2095005.
- WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. Geneva: World Health Organization; 2010. 5, LABORATORY METHODS FOR DIAGNOSIS OF HIV INFECTION IN INFANTS AND CHILDREN. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138552/
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100B.) HUMAN IMMUNODEFICIENCY VIRUS-1. Available from: https://www.ncbi.nlm.nih.gov/books/NBK304351/
- Maartens, G., Celum, C., & Lewin, S. R. (2014). HIV infection: epidemiology, pathogenesis, treatment, and prevention. The Lancet, 384(9939), 258–271. doi:10.1016/s0140-6736(14)60164-1
- Chrystie IL, Almeida JD. The morphology of human immunodeficiency virus (HIV) by negative staining. J Med Virol. 1988 Jul;25(3):281-8. doi: 10.1002/jmv.1890250305. PMID: 3171554.
- https://www.aids.gov.hk/pdf/g190htm/01.htm
- https://www.verywellhealth.com/hiv-aids-symptoms-4014373
- https://byjus.com/neet/hiv-diagram/
- https://viralzone.expasy.org/5183