Herpes simplex viruses (HSVs) are highly host-adapted viruses that can cause a variety of diseases in human hosts. (a) herpes simplex virus type 1 (HSV-1) and (b) herpes simplex virus type 2 (HSV-2) are the two forms of HSVs (HSV-2). Both types share DNA homology, antigenic determinants, tissue tropism, and illness symptoms, but their epidemiology is distinct. HSV-1 is transferred predominantly by contact with infected saliva, whereas HSV-2 is transmitted through sexual contact or vaginal tract infection from an infected mother to her newborn child.
Classification of human herpesviruses
| Subfamily | Scientific name | Common name | Site of latency |
| Alphaherpesvirinae | Human herpesvirus 1 | Herpes simplex virus type 1 | Neurons |
| Human herpesvirus 2 | Herpes simplex virus type 2 | Neurons | |
| Human herpesvirus 3 | Varicella zoster virus | Neurons | |
| Gammaherpesvirinae | Human herpesvirus 4 | Epstein–Barr virus | Lymphoid tissues |
| Human herpesvirus 8 | Kaposi’s sarcoma-related virus | — | |
| Betaherpesvirinae | Human herpesvirus 5 | Cytomegalovirus | Monocytes and lymphocytes in secretory glands |
| Human herpesvirus 6 | Human B cell lymphotrophic virus | Lymphoid tissue | |
| Human herpesvirus 7 | RK virus | Lymphoid tissue |
Human infections caused by human herpesviruses
| Herpes simplex virus (HSV)-1 | Acute herpetic gingivostomatitis, acute herpetic pharyngotonsillitis, herpes labialis, herpes encephalitis, eczema herpeticum, and herpetic whitlow |
| HSV-2 | Genital herpes, neonatal infection, and aseptic meningitis |
| Varicella zoster virus (VZV) | Chickenpox and herpes zoster |
| Epstein–Barr virus (EBV) | Infectious mononucleosis, EBV-induced tumors, such as Burkitt’s lymphoma, other B-cell lymphoma, and nasopharyngeal carcinoma. Duncan’s syndrome, lymphoproliferative syndrome, oral hairy leukoplakia. |
| Cytomegalovirus (CMV) | Congenital CMV infection, acquired CMV infection, CMV infection in immunocompromised patients, and CMV infection in immunocompetent adult hosts |
| HSV-6 | Roseola infantum, may also cause mononucleosis syndrome and lymphadenopathy |
| HSV-7 | Roseola infantum and febrile seizures in children |
| HSV-8 | Associated with Kaposi’s sarcoma, body cavity lymphoma, and Castleman disease |
What is Herpes Simplex Type 1?
- Type 1 herpes simplex virus (HSV-1) is a member of the subfamily Alphaherpesviridae and has a linear dsDNA genome. Primary and recurring vesicular eruptions, most commonly affecting the orolabial and vaginal mucosa, are caused by HSV-1.
- Orolabial herpes, herpetic sycosis (HSV folliculitis), herpes gladiatorum, herpetic whitlow, ocular HSV infection, herpes encephalitis, Kaposi varicelliform eruption (eczema herpeticum), and severe or chronic HSV infection are all possible manifestations of HSV-1 infection. The progression of HSV infection can be slowed with antiviral treatment.
- Alphaherpesviridae is a group of herpesviruses that includes herpes simplex virus type 1 (HSV-1). It has an icosahedral capsid between 100 and 110 nm in diameter and a spikey envelope, and is built from linear double-stranded DNA.
- Typically, epithelial cells are infected first during the pathogenesis of HSV-1 infection, and then the virus enters a dormant state, typically in neurons, before becoming active again.
- Primary and recurring vesicular eruptions, most commonly affecting the orolabial and vaginal mucosa, are caused by HSV-1.
- Orolabial herpes, herpetic sycosis (HSV folliculitis), herpes gladiatorum, herpetic whitlow, ocular HSV infection, herpes encephalitis, Kaposi varicelliform eruption (eczema herpeticum), and severe or chronic HSV infection are all possible manifestations of HSV-1 infection. The progression of HSV infection can be slowed with antiviral treatment.
Structure of Herpes simplex virus 1 (HSV-1)
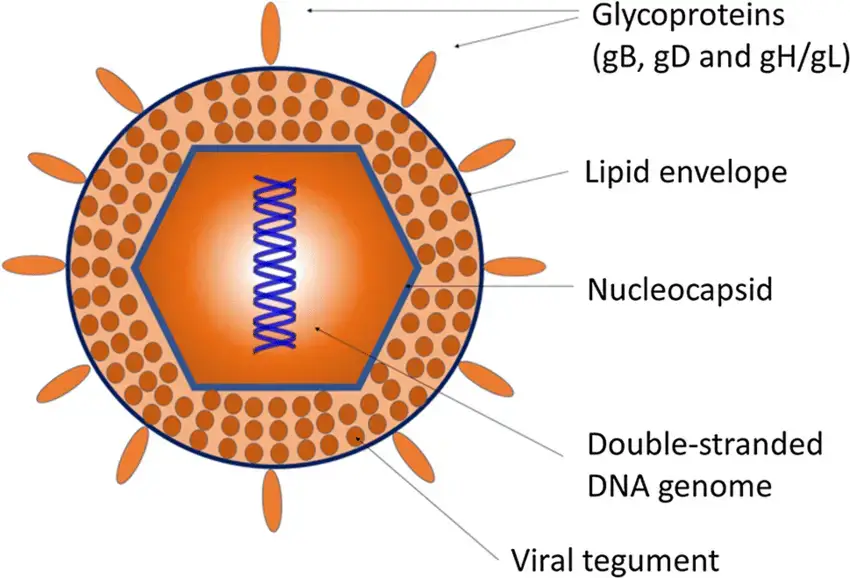
- The herpesviruses are about 150-200 nm in size, round, and have icosahedral symmetry.
- The nucleocapsid is comprised of the icosahedral protein capsid (average diameter 100 nm), which is composed of 162 hollow hexagonal and pentagonal capsomeres, and the double stranded DNA genome (125-240 kbp nucleotides), which is housed in the electron-dense core.
- A lipoprotein envelope surrounds the nucleocapsid.
- The lipid component originates in the infected host cell’s nuclear membrane.
- In order to enter the host cell, viruses use spikes of viral glycoproteins, each 8 nm in length, that project from the trilaminar lipid host-derived envelope.
- At least eleven glycoproteins are encoded by HSV; these include (a) viral attachment proteins (gB, gC, gD, gH), (b) fusion proteins (gB), (c) structural proteins, (d) immune escape proteins (gE, and gI), and (e) other fractions.
- Mature virus particles have a lipid envelope produced from host cell membranes enclosing an amorphous proteinaceous layer called the tegument outside of the capsid.
- Enzymes such as VP16 and VHS (Viron Host Shut off) protein, both found in the tegument, inhibit protein synthesis in the cytoplasm of the host cell, allowing the virus to replicate its nucleic acid.
Genome of Herpes simplex virus 1 (HSV-1)
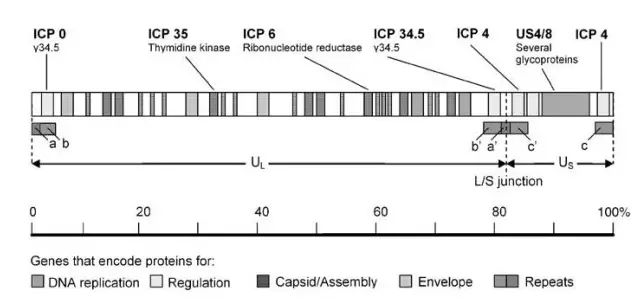
- The HSV-1 genome is a single, linear molecule of approximately 152,000 base pairs (bp) double-stranded DNA.
- It is separated into two distinct parts, long (UL) and short (US).
- There are brief areas of repetitive sequence (a/b/c and a’/b’/ c’) near the ends of the genome and between the L and S segments.
- As DNA is replicated, the L and S segments invert at a rapid rate, resulting in four isomers of the genome.
- The four occur with equal frequency in the majority of populations of HSV-1 wild type. There are 74 proteins encoded by the genome.
- The majority of genes encoding proteins are found in the L or S regions, and their names reflect their location within L or S.
- US6 encodes glycoprotein D, a membrane glycoprotein involved in virus entry, whereas UL30 encodes the DNA-dependent DNA polymerase of the virus.
- Based on their function, HSV1-encoded proteins can be categorised into four major groups: proteins for (1) virus DNA replication and recombination; (2) transcriptional regulation of HSV-1 gene expression and modulation of the host cell; (3) capsid development and assembly; and (4) envelope proteins.
- Above is a schematic depiction of the sites of the genes encoding these proteins within the HSV-1 genome. Indicated as well are the genes that are changed or deleted to achieve tumor-specific targeting and replication.
Risk factors for HSV-1 infection
- Depending on the kind of HSV-1 infection, the risk factors for HSV-1 infection vary. Risk factors for orolabial herpes include any action that exposes an individual to the saliva of an infected patient, such as sharing drinkware or cosmetics, or mouth-to-mouth contact.
- In the context of an acute orolabial infection, close shaving with a razor blade is the most significant risk factor for herpetic sycosis.
- Herpes gladiatorum risk factors include participation in high-contact sports such as rugby, wrestling, mixed martial arts, and boxing.
- Risk factors for herpetic whitlow in the child population include thumb sucking and nail biting in the presence of orolabial HSV-1 infection, and medical/dental profession in adults (although HSV-2 most commonly causes herpetic whitlow in adults).
- Mutations in the toll-like receptor (TLR-3) or UNC-93B genes pose a significant risk for herpes encephalitis. The hypothesis is that these mutations impede interferon-based responses.
- The primary risk factor for eczema herpeticum is a dysfunctional skin barrier. This is a characteristic of atopic dermatitis, Darier disease, Hailey-Hailey disease, mycosis fungoides, and all kinds of ichthyosis. Mutations in the filaggrin gene, observed in atopic dermatitis and ichthyosis vulgaris, are also connected with the higher risk. The use of topical calcineurin inhibitors, such as pimecrolimus and tacrolimus, is a pharmaceutical risk factor for eczema herpeticum.
- Immunocompromised states, such as transplant recipients (solid organ or hematopoietic stem cells), HIV infection, or leukemia/lymphoma patients, are risk factors for severe or persistent HSV infection.
Epidemiology
It is hypothesised that roughly one-third of the world’s population has had symptomatic HSV-1 at some point in their lifetime. HSV-1 initiates primary infection in people who lack antibodies against HSV-1 or HSV-2. Non-primary initial infection is infection with one HSV subtype in patients who have antibodies against the other HSV type (i.e., HSV-1 infection in a patient with HSV-2 antibodies, or vice versa). Reactivation causes recurrent infection and manifests most typically as asymptomatic viral shedding.
In the United States, around 1 in 1000 babies are infected with the herpes simplex virus due to exposure during vaginal delivery. Women with persistent genital herpes have a limited probability of passing HSV vertically to their newborn. However, pregnant women who get a genital HSV infection are at a greater risk.
Herpes encephalitis is the largest cause of fatal encephalitis in the United States, and ocular HSV infection is a significant cause of blindness in the country.
Symptoms of HSV-1
Depending on the individual, HSV-1 can manifest as painful blisters or ulcers in or around the mouth. Additional frequent symptoms include:
- Fever
- Aching muscles
- Swollen glands
- Painful urination
In certain situations, patients infected with HSV-1 may exhibit no symptoms, making it difficult to diagnose the infection.
Transmission of Herpes simplex virus 1 (HSV-1)
- The herpes simplex virus type 1 (HSV-1) is primarily spread through oral secretions, such as saliva, as well as sores and surfaces near the mouth.
- It can also be passed on to the genital area through oral-genital contact, although this is less common. The greatest transmission risk is when active sores are present. Individuals who already have HSV-1 can still contract HSV-2, even though they can’t get reinfected with the same virus.
- HSV-2, on the other hand, is mainly transmitted sexually, through contact with genital or anal surfaces, skin, sores, or bodily fluids from an infected person. Even without symptoms, the virus can still be spread.
Replication of Herpes simplex virus 1 (HSV-1)
Herpes Simplex Virus 1 (HSV1) is a highly infectious virus that can cause serious infections in humans. This virus is well known for its ability to invade cells and replicate itself efficiently. The invasion of cells by HSV1 requires a complex series of steps that are critical for the virus’s replication cycle.
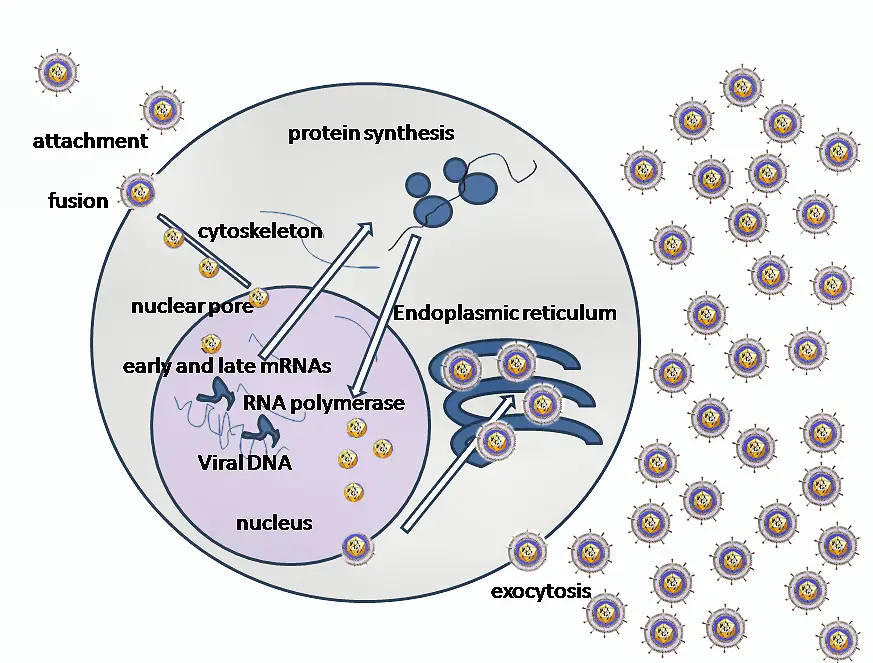
- Binding to Receptors: The first step in the invasion of cells by HSV1 is the binding of the envelope glyco-protein-C (gC) and/or gB to Heparan sulfate receptors. The virus then engages one of several co-receptors, including Herpes virus entry mediator A (HveA), through the binding of gD.
- Fusion and Capsid Delivery: The next step in the invasion process is the fusion of the viral envelope with the cell plasma membrane. This allows the viral capsid to be delivered into the cell cytoplasm. Along with the capsid, viral proteins VHS and VP16 are also released into the cytoplasm.
- Viral Propelling to Nucleopore: The incoming viral capsids are then propelled to the nucleopore where they enter the nucleus. Once in the nucleus, the viral capsids get disintegrated and only the DNA is released into the nucleus.
- Viral Transcription and Replication: The viral genome is then uncoated, allowing for viral transcription and replication to occur in the nucleoplasm. There are two main phases of transcription – early, which takes place prior to genome replication, and late, which takes place upon replicated genomes in virus replication compartments formed in the infected cell nucleus.
- Classes of mRNA: During transcription, three distinct classes of mRNAs are produced: Alpha, Beta, and Gamma. These classes are regulated in a coordinated, cascade fashion. The Alpha or Immediate-early (IE) genes contain the major transcriptional regulatory proteins and their production is required for the transcription of the Beta and Gamma gene classes.
- Beta Proteins: The Beta proteins include the enzymes required for replication of the viral genome, such as a DNA polymerase, a single-strand DNA-binding protein, a primosome or helicase-primase, an origin-binding protein, and a set of enzymes involved in DNA repair and in deoxynucleotide metabolism.
- Gamma Proteins: Viral DNA synthesis begins shortly after the appearance of the Beta proteins. The temporal program of viral gene expression ends with the appearance of the Gamma or late proteins, which constitute the structural proteins of the virus.
- Circularizing the Genome: The linear 153Kb pair genome circularizes shortly after the infection of susceptible host cells. It then enters a rolling circle mode of DNA replication, generating branched concatameric DNA, which is then cleaved to release linear ds DNA.
- Primary Infection and Virion Assembly: Viral transcription and DNA replication occur in the nucleus. The virus then assembles and exits from epithelial cells in the skin, causing a primary infection. The virion acquires its envelope by budding through the nuclear membrane.
Herpes Simplex Virus 1 (HSV1) is a highly infectious virus that can invade cells and replicate efficiently. The invasion process requires a series of complex steps, including binding to receptors, fusion and capsid delivery, viral propelling to nucleopore, viral transcription and replication, and virion assembly. Understanding the mechanisms behind HSV1 invasion and replication is critical for developing effective treatments and prevention strategies.
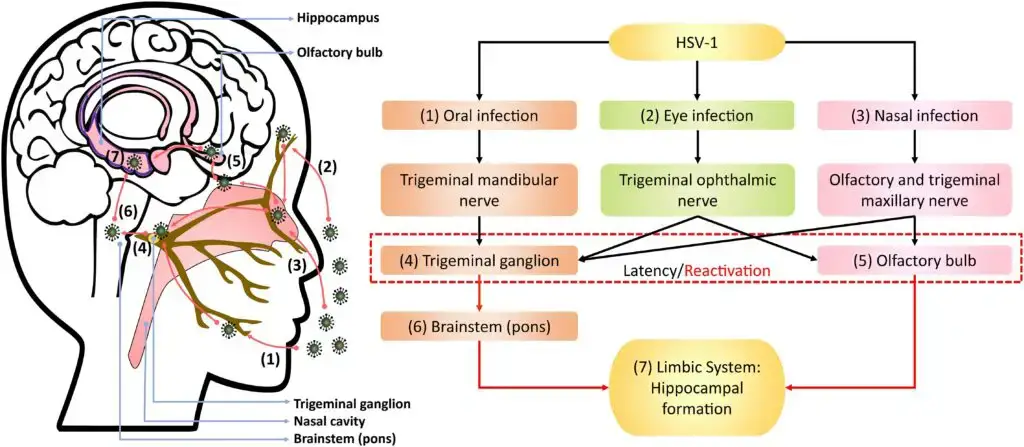
Pathogenesis of Herpes simplex virus 1 (HSV-1)
Herpes Simplex Virus 1 (HSV-1) is a highly contagious virus that affects millions of people worldwide. It is primarily spread through oral contact, such as kissing or sharing saliva, and can also be acquired through sexual activity. This article explores the basics of HSV-1 and how it affects the body.
1. The Attachment and Infection of HSV-1
The virus infects epithelial cells and starts with the attachment of viral particles to susceptible cells. The virions interact with cell-surface receptors through glycoproteins that project from the viral envelope. The typical lesion produced by HSV-1 is a vesicle, a ballooning degeneration of intra-epithelial cells that contains infectious fluid. The base of the vesicle contains multinucleate cells, and the infected nuclei contain eosinophilic inclusion bodies. Over time, the roof of the vesicle breaks down, forming an ulcer. This process occurs rapidly on mucous membranes and non-keratinizing epithelia and is slower on the skin.
2. The Role of Natural Killer Cells
Natural killer (NK) cells play a significant role in early defenses against HSV-1 by recognizing and destroying infected cells. This is crucial in preventing the spread of the virus and limiting the severity of the infection.
3. Three Unique Biological Properties of HSV-1
HSV-1 displays three unique biological properties: neurovirulence, latency, and reactivation. After the initial infection at the local site, the virus invades the local nerve ending, travels to the dorsal root ganglia, replicates further, and then enters a latent state. Most primary HSV-1 infections are mild, and many are asymptomatic. In the latent stage, the virus does not replicate, except for a small RNA encoded by a latency-associated viral gene, which maintains the latent infection and prevents cell death. The processes behind reactivation are not yet fully understood, but it is believed that HSV DNA travels along the nerve axon back to the nerve ending, where it may infect epithelial cells.
4. Factors Influencing Recurrence
Reactivation does not always result in a visible lesion, and there may be asymptomatic shedding of the virus. The factors influencing the development of recrudescent lesions are not yet clearly identified, but an increase in CD8+ T suppressor lymphocyte activity and a temporary decrease in immune effector cell function, particularly delayed hypersensitivity, may enhance the spread of HSV-1. Certain triggers, such as ultraviolet light, fever, trauma, and stress, are commonly associated with herpes recurrence. The interval between the stimulus and the appearance of a clinically obvious lesion is 2 to 5 days.
In conclusion, Herpes Simplex Virus 1 is a highly contagious virus that affects millions of people worldwide. Understanding the mechanisms behind its spread and impact on the body is crucial in preventing the spread of the virus and limiting its severity. While much is still unknown about HSV-1, ongoing research continues to shed light on this complex virus.
Clinical manifestations of Herpes simplex virus 1 (HSV-1)
HSV-1 is capable of causing a vast array of clinical phenomena. These include herpes labialis, herpes encephalitis, eczema herpeticum, and herpes whitlow.
1. Herpetic gingivostomatitis, acute
Acute herpetic gingivostomatitis is the symptom of primary HSV-1 infection in children aged six months to five years. The infectious agent is saliva from an infected child or adult. This illness has an incubation period of three to six days. The disease has a rapid start and a high temperature. Gingivitis is the most significant manifestation, characterised by prominent lip edoema and erythematous, friable gums. There are vesicular lesions on the oral mucosa, tongue, and lips. These lesions eventually rupture and consolidate, leaving ulcerated plaques behind. The duration of the acute condition is 5–7 days, and the duration of the symptoms is 2 weeks. There may be continued viral shedding from the saliva for at least three weeks.
2. Herpetic pharyngotonsillitis acute
In adults, pharyngitis and tonsillitis are more frequently caused by HSV-1 than gingivostomatitis. The symptoms of this illness are fever, lethargy, headache, and sore throat. Tonsil and posterior pharyngeal vesicular lesions typically rupture to produce ulcers. Lesions of the oral and labial mucosa are observed in less than 10% of individuals.
3. Herpes labialis
The most prevalent clinical manifestation of recurrent HSV-1 infection is herpes labialis. At the site of an infection, pain, burning, and tingling sensations are presenting symptoms. The typical lesion is an intraepidermal vesicle that eventually turns pustular and ulcerated. Typically, two or fewer recurrences occur annually in the majority of individuals. However, some patients experience monthly recurrence.
4. Herpes encephalitis
Herpes encephalitis is an acute febrile illness caused by HSV-1. This may be an indication of a primary or recurring viral infection. The infection’s onset may be gradual or sudden. During an acute disease, focal neurological symptoms such as seizures, hemiparesis, aphasia, and paresthesia may be observed. In certain cases, the infection may advance swiftly from stupor to coma to death without causing any localised neurological symptoms.
5. Eczema herpeticum
Active eczema is observed in children with eczema herpeticum. On previously eczematous regions with severe ulceration, vesicular lesions occur quickly. The infection may then spread to the adrenal glands, liver, and other organs via the blood, resulting in deadly consequences.
6. Infected whitlow
Herpetic whitlow is an infection of the finger caused by the virus entering the skin through abrasions or wounds. The illness is common among physicians and nurses who are exposed to patients with HSV infection or genital infection. Typically, vesicular lesions appear on the skin of the finger, but they may also appear on the skin of the head and neck.
Laboratory Diagnosis of Herpes simplex virus 1 (HSV-1)
- Culture: For virus isolation, tissue cultures are inoculated. HSV is simple to culture, and cytopathic effects often manifest within 3–5 days. The virus can develop rapidly in fibroblast and epithelial cell cultures, where it causes typical cell grounding and ballooning. The agent is then detected by a neutralisation test or immunofluorescence staining using particular antiserum.
- Cytopathology: Cytopathology involves the detection of multinucleated giant cells in vesicle base scrapings stained with Giemsa or Wright’s stain, often known as Tzanck smear preparation. On the stained smear, the presence of characteristic large cells or cow dry type A intranuclear inclusion bodies is diagnostic of HSV infection.
- Antigen detection: By direct fluorescent antigen detection and direct enzyme immunoassay, the antigen can be identified in vesicle fluid, tissue smear, and biopsy.
- Antibody detection: Antibodies manifest 4–7 days after infection and reach their peak in 2–4 weeks. ELISA, IFT, and Complement fixation tests can diagnose primary infection by measuring the presence of IgM or the growing titre of IgG. Glycoprotein G-based serologic assays can discriminate between HSV-1 and HSV-2.
- Molecular Diagnosis: PCR is the most sensitive method for detecting HSV DNA and can distinguish between HSV-1 and HSV-2.
Treatment of Herpes simplex virus 1 (HSV-1)
Acyclovir boasts a superior therapeutic benefit and established effectiveness. This nucleoside analog is first monophosphorylated by the herpes simplex virus (HSV) thymidine kinase, and then transformed into its triphosphate form through the actions of cellular kinases. The HSV polymerase effectively incorporates the acyclovir triphosphate into viral DNA, thereby hindering further chain extension. In addition to acyclovir, other medications such as valacyclovir and vidarabine are utilized, which obstruct DNA synthesis.
Prevention and Control of Herpes simplex virus 1 (HSV-1)
No vaccine exists for herpes simplex virus (HSV) infection. The spread of the virus can be reduced through:
- Addressing over-crowding
- Practicing good personal hygiene
- Acquiring knowledge on the infectious stages
- Implementing condom use.
FAQ
What is herpes simplex virus 1 (HSV-1)?
Herpes simplex virus 1 (HSV-1) is a viral infection that primarily affects the oral area (lips, mouth, gums). It’s commonly known as oral herpes or cold sores.
How is HSV-1 transmitted?
HSV-1 is primarily transmitted through close personal contact such as kissing or sharing food and drinks with an infected person. It can also be transmitted through oral sex.
What are the symptoms of HSV-1?
Common symptoms of HSV-1 include painful blisters or sores in the mouth or around the lips, fever, sore throat, and swollen lymph nodes.
Is there a cure for HSV-1?
Currently, there is no cure for HSV-1. However, antiviral medications can help reduce the frequency and severity of outbreaks and also help to prevent transmission.
Can HSV-1 be prevented?
HSV-1 can be prevented by avoiding close personal contact with infected individuals, not sharing food and drinks, and using barrier methods (such as condoms) during oral sex. Vaccination is also available to help prevent infection with HSV-1.
References
- Cliffe, A., Chang, L., Colgrove, R., & Knipe, D. M. (2014). Herpes Simplex Virus. Reference Module in Biomedical Sciences. doi:10.1016/b978-0-12-801238-3.00080-5
- Wiedbrauk, D. L. (2010). Herpes Simplex Virus. Molecular Diagnostics, 453–460. doi:10.1016/b978-0-12-369428-7.00037-9
- Everts, Bart & Poel, Henk. (2005). Everts, B and van der Poel, HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther 12: 141-161. Cancer gene therapy. 12. 141-61. 10.1038/sj.cgt.7700771.
- Saleh D, Yarrarapu SNS, Sharma S. Herpes Simplex Type 1. [Updated 2022 Aug 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482197/
- https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus