Curious about gymnosperms and their role in the plant kingdom? Whether you’re wondering, “Is a pine tree a gymnosperm?” or “What’s the difference between gymnosperms and angiosperms?”, this guide unlocks the answers. Gymnosperms (from the Greek for “naked seeds”) are a group of seed-producing plants that include conifers, cycads, and ginkgos. Unlike angiosperms (flowering plants like the petunia, which is an angiosperm), gymnosperms do not produce flowers or fruits. Instead, their seeds develop openly on structures like cones, answering “Do gymnosperms have ovules?” with a resounding yes—ovules are exposed on cone scales.
A key innovation in gymnosperm evolution was the development of seeds, which allowed them to thrive in diverse environments. But do gymnosperms have vascular tissue? Absolutely! Like angiosperms, they possess xylem and phloem, making them vascular plants. While they lack flowers (“Does gymnosperms have flowers?”), they reproduce via pollen dispersed by wind, fertilizing ovules to create seeds. This process addresses “How do gymnosperms reproduce?” and highlights their reliance on cones for reproduction.
So, what’s the difference between gymnosperms and angiosperms? Beyond seed exposure, gymnosperms typically have needle-like leaves and thrive in cooler climates, while angiosperms dominate with floral diversity. Common examples like conifers (e.g., pine trees) underscore their ecological importance. Whether exploring “Are ferns gymnosperms?” (no—ferns are seedless) or dissecting “What are the main components of a mature gymnosperm seed?” (embryo, nutritive tissue, seed coat), this guide clarifies the unique traits of these ancient plants. Dive deeper to uncover why gymnosperms remain vital to ecosystems and evolutionary studies alike!
What is the Gymnosperm?
- Gymnosperms are a class of seed-producing vascular plants distinguished by seeds not encased within an ovary or fruit, therefore known as “naked seeds.”
- With a fossil record going back to the late Paleozoic Era, some 390 million years ago, they are among the earliest extant seed plants.
- There are four main divisions among gymnosperms:
- Pinophta, or conifers,
- Cycadophyta, often known as cycads
- Ginkgophyta, also known as Ginkgo biloba
- Gnetophyta, comprising Gnetum, Ephedra, and Welwitschia, also includes
- Their life cycle shows a dominating sporophyte generation; the gametophyte generation is diminished and depends on the sporophyte.
- Mostly evergreen, gymnosperms are woody plants—that is, trees and shrubs. Their leaves are either scale-like or needle-like, suited to save water.
- In gymnosperms, reproduction is the creation of cones, or strobili, where female cones bear ovules and male cones create pollen. Usually, pollen is wind-mediated.
- Economically, gymnosperms are major suppliers of paper, lumber, resins, and other goods. Lumber companies make great use of species including pines, firs, and spruces.
- Ecologically, they are vital for habitat supply, carbon sequestration, and as pioneer species in many ecosystems.
- Gymnosperms help one better grasp plant evolution, particularly the change from seedless to seed-bearing plants.
Gymnosperm definition
Gymnosperms are vascular, seed‑producing plants whose ovules (seeds) develop exposed (“naked”) on cone scales rather than enclosed within an ovary
Habitat of Gymnosperms
- Gymnosperms show adaptation to several environmental circumstances by occupying a broad spectrum of habitats throughout several climatic zones.
- In boreal woods (taiga), gymnosperms—especially conifers—rule the scene. Cold temperatures, brief growth seasons, and nutrient-starved soils define these areas.
- Gymnosperms abound in mixed forests in temperate zones, usually coexisting with angiosperms. They are tuned to seasonal fluctuations in temperature and precipitation.
- Some gymnosperms such as cycads flourish in tropical and subtropical climates. These places have high humidity and warm temperatures suited for their development.
- Gymnosperms like Ephedra species are suited to live in dry and semi-arid conditions by means of little water. Their deep root systems and little leaf surface area define them.
- Gymnosperms like some species of Abies and Picea abound in alpine and subalpine zones. Low temperatures, strong UV exposure, and short growth seasons define these places.
- Gymnosperms are mostly found in India’s Himalayan area, where the temperature supports their development. Native to these regions are species include Picea smithsian and Abies pindrow.
- Usually missing from aquatic ecosystems, gymnosperms are scarce in very cold arctic areas or very hot deserts, suggesting their inclination for certain terrestrial habitats.
Characteristics of Gymnosperms
- Gymnosperms are woody perennial plants that produce seeds. They are known as “naked seeds” because their seeds are not encased in an ovary or fruit.
- They have characteristics with both angiosperms (flowering plants) and cryptogams (non-seed plants), making them an intermediate category.
- The reproductive organs of gymnosperms are cones or strobili, and the seeds grow on the inside of scale leaves that are spirally organized. Gymnosperms do not have flowers or fruits.
- Gymnosperms are unable to develop fruit because they lack an ovary; instead, their seeds are left out in the open.
- They can survive in dry environments thanks to their severe xerophytic adaptations, which include needle-like leaves with thick cuticles and depressed stomata.
- Aquatic habitats do not support gymnosperms, which are only found on land.
- Both stems and roots experience secondary development, which thickens these organs and adds to the woody characteristics of these plants.
- Without the protective covering that angiosperms have, seeds are usually exposed on the surface of cone scales after developing from ovules.
- Gymnosperms frequently exhibit polyembryony, in which a single fertilized egg can give rise to many embryos, albeit typically only one of them grows.
- Oogamous sexual reproduction combines a huge, immobile egg with a smaller, motile sperm.
- Cones that are male and female are different; the former have megasporophylls with megasporangia, while the latter have microsporophylls with microsporangia.
- In gymnosperm seeds, the endosperm acts as a food store to aid in the embryo’s growth.
- Because of their adaptations to these conditions, gymnosperms are mostly found in areas with snow as the main supply of water, such as cold climes.
- Gymnosperm pollination is mostly wind-mediated, meaning that pollen grains do not require a stigma to reach the ovules.
- Gymnosperms are unisexual plants that can be either dioecious (having only one type of cone) or monoecious (having both male and female cones).
- They don’t have the angiosperm-specific mechanisms of triple fusion and double fertilization.
- Because gymnosperms do not depend on animals for pollination, their reproductive organs do not have colored sepals and petals.
- The suspensor, a mechanism that forces the embryo into the seed’s nutritive tissue, is where the embryo develops in gymnosperms.
Morphology and Anatomy of Gymnosperms
- Mostly medium to big woody plants, Gymnosperms include shrubs and trees. Sporophytic and subdivided into roots, stems, and leaves, the plant body
- Usually connected with mycorrhizal fungus, the deep taproot of the root system improves nutrient and water absorption.
- Usually standing straight, stems can be branched or unbranched. For example, Pinus has a branching stem that may reach heights of 10–50 meters; Cycas has an unbranched columnar stem.
- Usually suited to cut water loss, leaves are either needle-like or scale-like. Often spirally organized and maybe evergreen, they help the plant to be xerophytic.
- Gymnosperms produce yearly rings by secondary growth in stems and roots. Conjoint, collateral, endarch, open, and conjoint vascular bundles help to effectively move water and nutrients.
- Tracheids, long cells helping in water conduction, make up the xylem mostly. Phloem lacks partner cells; instead, sieve cells abound.
- Commonly present in the cortex and xylem, resin ducts protect against herbivores and infections.
- To reduce water loss, leaves can show xeromorphic traits including depressed stomata and thick cuticles. Some species decrease leaves to scales, therefore lowering transpiration even further.
- Usually undifferentiated, the mesophyll in leaves helps the lateral circulation of water and nutrients by surrounding vascular bundles with transferable tissue.
- Reproductive structures arrange themselves in cones or strobili. Female cones generate megaspores; male cones generate microspores. Usually unisexual, these structures let plants be monoecious or dioecious.
- On the surface of megasporophylls, seeds are exposed missing an enclosing ovary. Gymnosperms stand out from angiosperms in this way.
- Developing at the end of a suspensor, the embryo pushes itself into the seed’s nutritious tissue. Though usually only one embryo develops, polyembryony—the production of many embryos from a single fertilized egg—is common.
Plant body of gymnosperms
- Gymnosperms’ diploid sporophyte is their main and most visible phase in their life cycle. ,
- Usually woody perennials, gymnosperms include shrubs and medium-height trees. Particularly noteworthy are species such as Sequoiadendron giganteum, which may reach heights of 100 meters.
- Three primary organs—roots, stems, and leaves—make up the plant body. This variation helps the plant to be generally functioning and to flourish.
- Usually connected with mycorrhizal fungus, the deep taproot of the root system improves water and nutrient absorption.
- Usually upright, stems can be branched or unbranched. For example, Pinus has a branching stem that could reach heights of 10–50 meters, while Cycas has an unbranched columnar stem.
- Usually scale-like or needle-like, leaves are designed to minimise water loss. Often organized spirally and maybe evergreen, they help the plant to be xerophytic.
- Gymnosperms create yearly rings by secondary development in stems and roots. Conjoint, collateral, endarch, open, and conjoint vascular bundles help to effectively move nutrients and water.
- Tracheids, elongated cells helping in water conduction, make up most of the xylem. Phloem lacks partner cells; instead, sieve cells are seen.
- Commonly present in the cortex and xylem, resin ducts protect against herbivores and pathogens.
- To reduce water loss, leaves typically show xeromorphic traits including depressed stomata and thick cuticles. Some species have reduced leaves to scales, therefore lowering transpiration even further.
- Usually undifferentiated, the mesophyll in leaves helps the lateral transfer of water and nutrients by surrounding vascular bundles with transfusion tissue.
- Reproductive structures fall into cones or strobili. Female cones create megaspores; male cones create microspores. Usually unisexual, these structures are seen in plants either monoecious or dioecious.
- Lacking an enveloping ovary, seeds are exposed on the surface of megasporophylls. Gymnosperms stand apart from angiosperms in this feature.
- The embryo grows toward the end of a suspensor, which forces it into the seed’s nutritious tissue. Although usually only one embryo develops, polyembryony—the growth of many embryos from a single fertilized egg—is common.
Roots of gymnosperms
- Usually having a taproot system, gymnosperms have a central, main root that extends downward with lateral branches off. For water and nutrients, this system offers stability and access to more profound soil layers.
- Gymnosperms can have two or more xylem poles as their roots show diarch to polyarch vascular configurations. These roots have exarch xylem; the protoxylem is found at the periphery and the metxylem in the core.
- Apart from their main root system, gymnosperms might create specialized root forms:
- Mycorrhizal roots develop symbiotic relationships with fungus to improve nutrient absorption, particularly phosphorus, and water absorption.
- Found in genera like Cycas, coralloid roots lack root hairs and caps, are uneven, dichotomously branching, Contributing to nitrogen fixation are nitrogen-fixing cyanobacteria such Anabaena and Nostoc.
- Development of the root system is shaped by surroundings. For example, the life and development of a plant depend critically on mycorrhizal connections in nutrient-starved soils.
Stem of gymnosperms
Gymnosperm stems are often upright and lignified, functioning as the principal support structure for the plant. They may be unbranched, as exemplified by Cycas, or branching, as demonstrated by Pinus and Cedrus.
The stem architecture differs among gymnosperms:
- Cycas – Exhibits a cylindrical, unbranched stem containing a central core of vascular tissue surrounded by parenchyma and sclerenchyma. The epidermis frequently becomes disrupted due to enduring leaf bottoms, and the vascular bundles are conjoint, collateral, open, and endarch.
- Pinus – Exhibits a ribbed trunk with a wide parenchymatous cortex. The vascular tissue comprises distinct collateral and open vascular bundles organized in a ring, interspersed with medullary rays. Resin ducts are located in the cortex and vascular strands.
Secondary growth is prevalent in gymnosperms, resulting in an expansion in stem diameter with time. This growth leads to the development of yearly rings, seen in species like as Pinus.
The vascular cambium of the stem facilitates secondary growth through the production of secondary xylem (wood) and phloem. In mature stems, the vascular cambium is accountable for the development of these tissues, enabling the plant’s increase in diameter.
Resin ducts are a prominent characteristic in gymnosperm stems, particularly in species such as Pinus. These ducts excrete resin, functioning as a defensive strategy against herbivores and pathogens.
The cortex of the stem of gymnosperms frequently has parenchymatous cells with thin walls that store starch, calcium oxalate crystals, and mucilage. These components enhance the plant’s metabolic processes and structural integrity.
In certain gymnosperms, like Cycas, the stem has a distinctive architecture including a core vascular cylinder around by an expansive cortex. The vascular bundles are organized in a circular formation, and the stem’s surface is uneven due to the presence of leaf bases.
Medullary rays in the stem enhance lateral transfer of water and nutrients between the xylem and phloem, improving the efficiency of the plant’s vascular system.
Leaves of gymnosperms
Gymnosperms have different leaf morphologies; microphyllous and megaphyllous types abound. Usually deciduous and protective of the bigger foliage leaves, microphyllous leaves are tiny, scale-like and usually lack lateral veins.
Larger, well-developed, either simple or compound, megaphyllous leaves can range in size from minute scale leaves to several feet long, as shown in Cycas. A characteristic of gymnosperms, the leaf-gap in the stem stele results from the vascular supply to these leaves.
Leaves could be positioned in rosettes at the top of the stem, in clusters, or spirally. Usually scale-like or needle-like, leaves of conifers are evergreen, which helps them to adapt to demanding surroundings.
Usually coated in a thick cuticle to reduce water loss, gymnosperms’ leaf epidermis Many times submerged below the epidermal surface, stomata lower transpiration rates even further.
The mesophyll is homogenous; all photosynthetic paredyma cells resemble palisade paredyma and have little intercellular gaps.
For water conduction, gymnosperm leaves’ vascular structure consists of tracheids and xylem parenchyma; for food movement, sieve cells form.
Common in the mesophyll, resin ducts have both physiological and defensive roles.
Mostly apical, leaf development is aided by marginal meristems.
Adapted to xerophytic circumstances, gymnosperm leaves show traits including depressed stomata and thick cuticles to help to save water.
Gymnosperms’ leaf form reflects their position among some of the earliest seed-producing plants on Earth and their evolutionary adaptations to various environmental niches.
Classification of Gymnosperms
The classification of gymnosperms is complex due to the existence of numerous fossil and living forms, which has led to various taxonomic systems over time. This intricate classification reflects the evolutionary relationships and morphological characteristics of these plants. Several researchers have contributed to the classification of gymnosperms, with significant systems developed in the 20th century.
- Early Classifications:
- In 1917, Counter and Chamberlain established a classification that included seven orders:
- Cycadofilicals
- Bennettitales
- Cycadales
- Cordaitales
- Ginkgoales
- Coniferales
- Gnetales
- In 1917, Counter and Chamberlain established a classification that included seven orders:
- Chamberlain’s Division:
- In 1934, Chamberlain proposed a classification of gymnosperms into two main classes, each further divided into various orders:
- Class: Cycadophyta (divided into three orders):
- Cycadofilicales
- Cycadeiodales
- Cycadales
- Class: Coniferophyta (divided into four orders):
- Cordaitales
- Ginkgoales
- Coniferales
- Gnetales
- Class: Cycadophyta (divided into three orders):
- In 1934, Chamberlain proposed a classification of gymnosperms into two main classes, each further divided into various orders:
- Arnold’s Phyla (1948):
- Arnold classified gymnosperms into three phyla:
- Phylum: Cycadophyta (comprising three orders):
- Pteridospermales
- Cycadeoidales
- Cycadales
- Phylum: Coniferophyta (containing four orders):
- Cordaitales
- Coniferales
- Taxales
- Ginkgoales
- Phylum: Chlamydospermophyta (including two orders):
- Ephedrales
- Gnetales
- Phylum: Cycadophyta (comprising three orders):
- Arnold classified gymnosperms into three phyla:
- Andrew’s Classification (1961):
- Andrew proposed a classification that included six divisions:
- Pteridospermatophyta
- Cycadohyta
- Coniferophyte
- Ginkgohyta
- Gnetophyta
- Gymnosperms of uncertain affinities
- Andrew proposed a classification that included six divisions:
- Sporne’s Classification (1965):
- K.R. Sporne categorized gymnosperms into three divisions based on the Pilger and Melchior (1954) classification, further detailing the orders:
- A. Cycadopsida
- Order 1: Pteridospermales (seven families including Lyginopteridaceae, Medulosaceae, Calamopteridaceae, Glossopteridaceae, Peltospermaceae, Corystospermaceae, Caytoniaceae)
- Order 2: Bennettitales (three families: Williamsoniaceae, Wielandiellaceae, Cycadeoideaceae)
- Order 3: Pnetoxylaes (one family: Pentoxylaceae)
- Order 4: Cycadales (two families: Cycadaceae, Nilssoniaceae)
- B. Coniferopsida
- Order 1: Cordaitales (three families: Ertophytaceae, Cordaitaceae, Poroxylaceae)
- Order 2: Coniferales (nine families including Lebachiaceae, Vitziaceae, Palissyaceae, Pinaceae, Taxodiaceae, Cupressaceae, Podocarpaceae, Cephalotaxaceae, Araucariaceae)
- Order 3: Taxales (one family: Taxaceae)
- Order 4: Ginkgoales (two families: Trichoptyaceae, Ginkgoaceae)
- C. Gnetopsida
- Order 1: Gnetales (three families: Gnetaceae, Welwitschiaceae, Ephedraceae)
- A. Cycadopsida
- K.R. Sporne categorized gymnosperms into three divisions based on the Pilger and Melchior (1954) classification, further detailing the orders:
- Modern Classification:
- In contemporary systems, gymnosperms are primarily divided into four main orders:
- A. Cycadales
- Characterized as dioecious, with separate male and female plants, originating in the Triassic period. They are both woody and feature pinnately compound leaves, with large cones composed of fertile leaves. Examples include Cycas and Zamia.
- B. Ginkgoales
- Represented by the living species Ginkgo biloba. They possess pycnoxylic wood and motile sperm, exhibiting dichotomous venation in their leaves.
- C. Coniferales
- Comprising sporophytic trees or shrubs with richly branched structures. These plants lack vessels, have monoxylic wood, and include both male and female cones. Examples are Sequoia, Pinus, and Taxus.
- D. Gnetales
- Serving as a connecting link between gymnosperms and angiosperms, they feature pycnoxylic wood and reproductive structures resembling those of flowering plants. Notable examples include Gnetum, Ephedra, and Welwitschia.
- A. Cycadales
- In contemporary systems, gymnosperms are primarily divided into four main orders:
Life Cycle of Gymnosperms
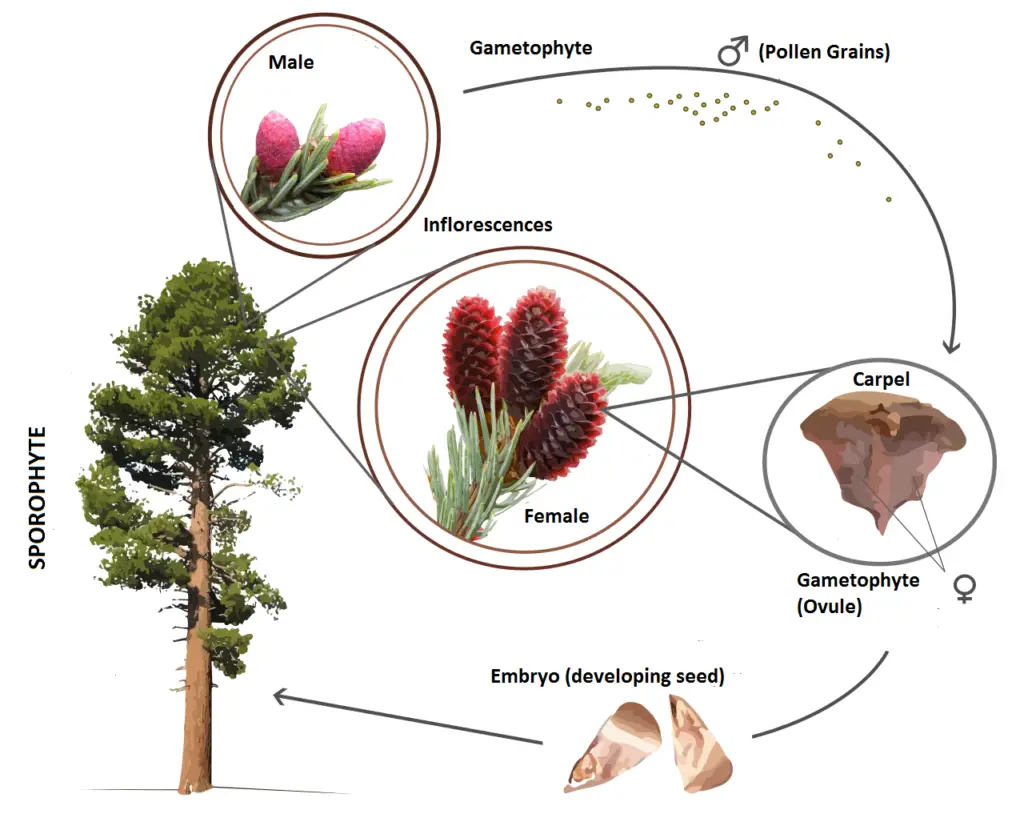
The life cycle of gymnosperms exemplifies the alternation of generations, a biological process where plants alternate between two distinct phases: the sporophyte phase, which is spore-bearing, and the gametophyte phase, which is gamete-bearing. This cycle intricately links the two generations, highlighting their roles in reproduction and development.
- Sporophyte Generation:
- The sporophyte phase is the dominant generation in gymnosperms, characterized by its diploid nature, containing two sets of chromosomes. This generation encompasses the mature plant, including roots, stems, cones, and leaves.
- Within the sporophyte generation, both male and female cones are produced. These cones may be located on the same plant or on separate plants. In instances where both types of cones are present on the same plant, the female cones (or strobili) are typically found at the upper part of the branches, while male cones are situated lower down.
- Male strobili produce haploid microspores through meiosis, whereas female cones undergo a similar process to produce megaspores.
- Gametophyte Generation:
- Following the production of microspores and megaspores, these undergo further development to yield haploid gametophytes. Specifically, the male gametophyte, known as the microgametophyte, develops from the microspores, while the female gametophyte, or megagametophyte, arises from the megaspores.
- These gametophytes have a short life span and play crucial roles in sexual reproduction. The male gametophyte develops into sperm cells, while the female gametophyte produces egg cells.
- It is important to note that the female gamete remains attached to the sporophyte during its development, receiving nutrients essential for the fertilization process.
- Pollination and Fertilization:
- Pollination occurs when male and female gametes unite, resulting in the formation of a diploid zygote. Pollination is facilitated by various agents, including wind, animals, and insects, ensuring genetic exchange and reproduction.
- As the zygote matures, it develops into a new diploid sporophyte, which is enclosed within a seed in the form of an embryo. This seed stage is vital for the plant’s survival, allowing it to withstand unfavorable conditions until it is ready to germinate.
- Seed Dispersal:
- Once the seeds reach maturity, they are dispersed through wind and rain, allowing them to spread to different locations. This dispersal is essential for reducing competition among seedlings and enhancing the chances of successful germination.
- When environmental conditions are favorable, the seeds germinate, giving rise to a new adult diploid plant. This newly formed plant will then initiate the life cycle anew, repeating the processes of sporophyte and gametophyte generation.
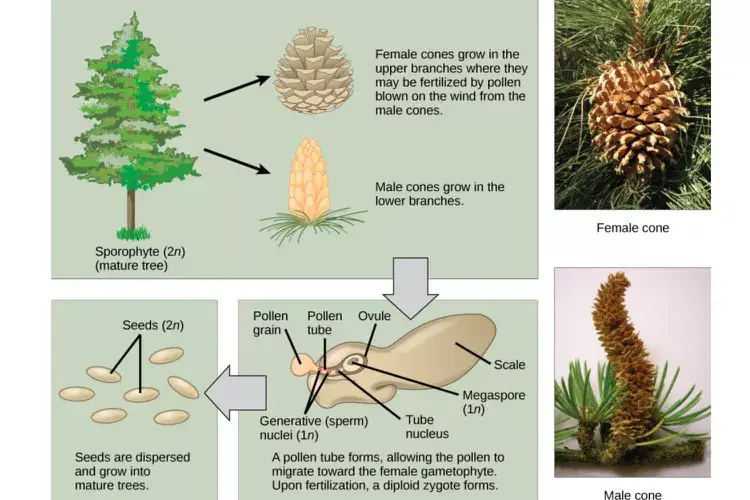
Reproduction of Gymnosperms
Gymnosperms reproduce sexually by producing seeds that are not encased in fruits, setting them apart from angiosperms.
- Formation of Male and Female Cones
- Gymnosperms produce two types of cones: male cones (microstrobili) and female cones (megastrobili).
- Male cones are typically smaller and located lower on the tree, while female cones are larger and positioned higher up.
- These cones serve as reproductive structures, housing the male and female gametophytes.
- Development of Male Gametophytes
- Within male cones, microsporangia undergo meiosis to produce haploid microspores.
- Each microspore develops into a pollen grain, which is the male gametophyte.
- Pollen grains are released into the air and carried by wind to female cones.
- Development of Female Gametophytes
- Female cones contain ovules, each with a megasporangium.
- Within each megasporangium, a megasporocyte undergoes meiosis to produce four haploid megaspores.
- Typically, only one megaspore survives and develops into the female gametophyte, which contains archegonia with egg cells.
- Pollination
- Wind carries pollen grains from male cones to female cones.
- Upon landing on a receptive ovule, the pollen grain germinates, forming a pollen tube that extends toward the egg cell.
- This process ensures cross-fertilization and genetic diversity.
- Fertilization
- Sperm cells travel through the pollen tube to reach the egg cell in the archegonium.
- One sperm cell fuses with the egg cell, resulting in a diploid zygote.
- Fertilization may occur months after pollination, depending on the species.
- Seed Development
- The fertilized zygote develops into an embryo within the ovule.
- The ovule matures into a seed, which includes the embryo, stored nutrients, and a protective seed coat.
- Seeds are released from the female cone when it opens, facilitating dispersal.
- Germination
- Upon landing in a suitable environment, seeds germinate, and the embryo develops into a new sporophyte.
- The young plant grows into a mature gymnosperm, completing the life cycle.
Economic importance of gymnosperms
Gymnosperms, particularly conifers, have significant economic importance due to their diverse applications across various industries.
- Wood and building materials:
- The main building material used in temperate climates, conifers supply all the softwood lumber used worldwide.
- Their wood finds great use in furniture design, building, and paper manufacture as a raw resource.
- Industry involving paper and pulp:
- For the paper business, gymnosperms are main suppliers of pulpwood.
- Strong and durable paper goods are best produced from the long fibers found in coniferous forests.
- Production of Resine and Turpentine:
- Turpentine and rosin are made from resins taken from gymnosperms—especially pines.
- In the chemical industry, these compounds find use as solvents, adhesives, and varnish manufacturing agents.
- Medical Use:
- Some gymnosperms have therapeutic effects. For instance, traditional medicine makes use of Ginkgo biloba leaves, which are supposed to provide cognitive advantages.
- Gymnosperm-derived compounds have anti-inflammatory and anticancer effects that find utility in medications.
- Landscape and decorative applications:
- Many gymnosperms, including firs, spruces, and pines, are grown ornately in gardens and parks.
- Their everlasting character and unique forms appeal for gardening.
- Environmental and ecological value:
- With gymnosperms dominating them, coniferous forests make up 31% of all forest planted area worldwide.
- These woods are quite important for carbon sequestration, so they help to slow down global warming.
- Maintaining ecological balance and stop of soil erosion depend on gymnosperms.
- Religious and Cultural Significance:
- Gymnosperms have symbolic connotations and are part of religious ceremonies in many different societies.
- For example, several Asian societies regard the Ginkgo tree as holy and usually place it next to temples.
- Conservation of Bi Diversity:
- Gymnosperms are habitats for many species and help to maintain biodiversity.
- Gymnosperm species conservation is essential as many are threatened and have special importance in their habitats.
Examples of gymnosperms
Pinus (Pine) – A genus within the Pinaceae family, comprising numerous species such as Pinus sylvestris (Scots pine) and Pinus longaeva (Bristlecone pine), known for their needle-like leaves and cones.
Cedrus (Cedar) – Includes species like Cedrus libani (Lebanon cedar) and Cedrus deodara (Himalayan cedar), characterized by their aromatic wood and large cones.
Abies (Fir) – Members of the Pinaceae family, such as Abies alba (European silver fir) and Abies balsamea (balsam fir), distinguished by their flat needle-like leaves and upright cones.
Juniperus (Juniper) – A genus in the Cupressaceae family, including species like Juniperus communis (common juniper), known for their berry-like cones and aromatic foliage.
Cupressus (Cypress) – Encompasses species such as Cupressus sempervirens (Mediterranean cypress), recognized for their scale-like leaves and small, woody cones.
Taxus (Yew) – A genus in the Taxaceae family, including species like Taxus baccata (European yew), notable for their red, fleshy arils and toxic foliage.
Cycas – A genus in the Cycadaceae family, such as Cycas revoluta (sago palm), characterized by their large, pinnate leaves and stout trunks.
Ginkgo biloba – The sole extant species in the Ginkgoaceae family, known for its fan-shaped leaves and resilience to urban pollution.
Ephedra – A genus in the Ephedraceae family, including species like Ephedra sinica, recognized for their jointed stems and medicinal properties.
Gnetum – A genus in the Gnetaceae family, comprising species like Gnetum gnemon, known for their broad leaves and edible seeds.
Welwitschia mirabilis – A unique species in the Welwitschaceae family, endemic to the Namib Desert, characterized by its two long, strap-like leaves and deep taproot.
Araucaria – A genus in the Araucariaceae family, including species like Araucaria araucana (monkey puzzle tree), known for their symmetrical branches and large cones.
Podocarpus – A genus in the Podocarpaceae family, such as Podocarpus macrophyllus (yew plum pine), recognized for their fleshy, berry-like cones and evergreen foliage.
Sciadopitys (Umbrella pine) – A genus in the Sciadopityaceae family, known for its whorled needle arrangement and unique reproductive structures.
Cephalotaxus (Plum yew) – A genus in the Taxaceae family, including species like Cephalotaxus harringtonia, characterized by their broad, dark green leaves and berry-like cones.
Dioon – A genus in the Zamiaceae family, such as Dioon edule, known for their stout trunks and large, pinnate leaves.
Encephalartos – A genus in the Zamiaceae family, including species like Encephalartos altensteinii, characterized by their large, arching leaves and stout trunks.
Macrozamia – A genus in the Zamiaceae family, such as Macrozamia communis, recognized for their large, pinnate leaves and stout trunks.
Bowenia – A genus in the Zamiaceae family, including species like Bowenia spectabilis, known for their large, glossy leaves and stout trunks.
Welwitschia mirabilis – A unique species in the Welwitschaceae family, endemic to the Namib Desert, characterized by its two long, strap-like leaves and deep taproot.
Gnetum gnemon – A species in the Gnetaceae family, known for its broad leaves and edible seeds.
Ephedra sinica – A species in the Ephedraceae family, recognized for its jointed stems and medicinal properties.
Taxus baccata – A species in the Taxaceae family, known for its red, fleshy arils and toxic foliage.
Araucaria araucana – A species in the Araucariaceae family, recognized for its symmetrical branches and large cones.
Morphology, Anatomy, and Reproduction of Cycas and Pinus
Cycas
1. Morphology
- General Structure: Cycas plants, often called cycads, resemble palm trees with their stout, unbranched trunks and large, pinnate leaves. The leaves are compound, with numerous leaflets arranged in a feather-like pattern.
- Leaf Characteristics: Leaves are typically thick and leathery, and are arranged in a rosette at the top of the stem. They can be up to several meters long.
- Reproductive Structures: Cycas plants produce cones that are separate for male and female plants. Male cones are cylindrical and bear microsporophylls, while female cones are larger, ovoid, and contain ovules.
2. Anatomy
- Stem: The stem of Cycas is a columnar structure with a central core of vascular tissue surrounded by a layer of parenchyma and sclerified tissue. The stem does not increase in girth significantly and remains relatively unbranched.
- Leaf Arrangement: Leaves are spirally arranged at the apex of the stem. Each leaf consists of a petiole and a rachis with numerous leaflets.
- Reproductive Anatomy: Male cones contain microsporangia within microsporophylls, where pollen grains develop. Female cones house ovules in their ovuliferous scales, and the fertilization occurs within these ovules.
3. Reproduction
- Pollination: Cycas is pollinated primarily by wind, although some species may use insects. Pollen grains are carried to the female cones.
- Fertilization: After pollination, the pollen grain germinates to form a pollen tube that travels to the ovule. Fertilization involves the fusion of male gametes with the female ovule.
- Seed Development: Fertilized ovules develop into seeds, which are often fleshy and are enclosed within the female cone. Seeds are typically large and can be dispersed by animals or wind.
Pinus
1. Morphology
- General Structure: Pinus, or pines, are evergreen trees with a tall, straight trunk and a conical crown. The tree is covered with needle-like leaves that are grouped in fascicles.
- Leaf Characteristics: Pine needles are slender and arranged in bundles of two to five. They are adapted to reduce water loss and withstand harsh environmental conditions.
- Reproductive Structures: Pines produce two types of cones: male cones (small, cylindrical) and female cones (larger, woody). Male cones are located towards the base of the tree, while female cones are typically found higher up.
2. Anatomy
- Stem: The stem of Pinus is woody and consists of a central pith surrounded by a vascular cambium, xylem, and phloem. The xylem provides structural support and is involved in water transport.
- Leaf Arrangement: Pine needles are arranged in clusters (fascicles) and are supported by a small petiole. Each needle is covered by a protective cuticle.
- Reproductive Anatomy: Male cones contain microsporangia on their scales, where pollen is produced. Female cones possess ovules on their cone scales, which develop into seeds after fertilization.
3. Reproduction
- Pollination: Pines are primarily wind-pollinated. Pollen from the male cones is released into the air and is carried to the female cones.
- Fertilization: Once pollen reaches the female cone, it germinates and forms a pollen tube that penetrates the ovule. Fertilization occurs within the ovule.
- Seed Development: Following fertilization, seeds develop within the female cone. Pine seeds are typically small and have wings that facilitate wind dispersal. The mature cones eventually open to release the seeds.
Comparison
- Morphology: Cycas plants have a more palm-like appearance with large, pinnate leaves and unbranched stems, while Pinus trees are coniferous with needle-like leaves and branched, woody trunks.
- Anatomy: Cycas has a more primitive structure with a central core of vascular tissue, while Pinus has a more advanced and complex woody structure with distinct xylem and phloem.
- Reproduction: Both Cycas and Pinus are seed-producing plants with separate male and female cones. However, Cycas cones are usually larger and more fleshy, whereas Pine cones are typically woody and have adapted to wind dispersal.
Angiosperms vs gymnosperms
| Feature | Angiosperms | Gymnosperms |
|---|---|---|
| Definition | Seed-producing flowering plants with seeds enclosed within an ovary. | Seed-producing non-flowering plants with unenclosed or “naked” seeds. |
| Seed Enclosure | Seeds are enclosed inside an ovary, usually forming a fruit. | Seeds are exposed, not enclosed; typically found on scales, leaves, or as cones. |
| Life Cycle | Generally seasonal; many are annual or biennial. | Mostly perennial and evergreen. |
| Endosperm | Triploid endosperm formed via double fertilization. | Haploid endosperm formed before fertilization. |
| Reproductive Structures | Flowers containing reproductive organs; can be unisexual or bisexual. | Cones (strobili) containing unisexual reproductive organs. |
| Leaves | Broad, flat leaves with various venation patterns. | Needle-like or scale-like leaves, often adapted to conserve water. |
| Cotyledons | Present; classified as monocots (one cotyledon) or dicots (two cotyledons). | Usually absent or not distinctly classified. |
| Wood Type | Hardwood; contains vessels in xylem for efficient water conduction. | Softwood; lacks vessels in xylem, relying on tracheids for water conduction. |
| Pollination Mechanism | Diverse; includes wind, water, and animal pollination. | Primarily wind pollination (anemophily). |
| Fertilization | Double fertilization resulting in a zygote and triploid endosperm. | Single fertilization; no double fertilization occurs. |
| Archegonia Presence | Absent; embryo sac develops without archegonia. | Present; female gametophyte contains archegonia. |
| Vascular Tissue | Xylem with vessels and phloem with companion cells. | Xylem lacks vessels; phloem lacks companion cells. |
| Examples | Mango, apple, wheat, rice, roses, lilies. | Pine, fir, spruce, cycas, ginkgo. |
- Wang, X., & Ran, J. H. (2014). Evolution and biogeography of gymnosperms. Molecular Phylogenetics and Evolution, 75, 24-40.
- Mathews, S. (2009). Phylogenetic relationships among seed plants: Persistent questions and the limits of molecular data. American Journal of Botany, 96(1), 228-236.
- Escapa, I. H., & Catalano, S. A. (2013). Phylogenetic analysis of Araucariaceae: Integrating molecules, morphology, and fossils. International Journal of Plant Sciences, 174(8), 1153-1170.
- Neale, D. B., & Wheeler, N. C. (2019). The Conifers: Genomes, Variation and Evolution. Springer Nature.
- Brenner, E. D., Stevenson, D. W., & Twigg, R. W. (2003). Cycads: evolutionary innovations and the role of plant-derived neurotoxins. Trends in Plant Science, 8(9), 446-452.
- Leslie, A. B., Beaulieu, J. M., Rai, H. S., Crane, P. R., Donoghue, M. J., & Mathews, S. (2012). Hemisphere-scale differences in conifer evolutionary dynamics. Proceedings of the National Academy of Sciences, 109(40), 16217-16221.
- Farjon, A. (2010). A handbook of the world’s conifers. Brill Academic Publishers.
Fragnière, Y., Bétrisey, S., Cardinaux, L., Stoffel, M., & Kozlowski, G. (2015). Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. Journal of Biogeography, 42(5), 809-820. - Gernandt, D. S., Willyard, A., Syring, J. V., & Liston, A. (2011). The conifers (Pinophyta). In Genetics, genomics and breeding of conifers (pp. 1-39). CRC Press.
- Wu, C. S., & Chaw, S. M. (2015). Evolutionary stasis in cycad plastomes and the first case of plastome GC-biased gene conversion. Genome Biology and Evolution, 7(7), 2000-2009.
https://courses.botany.wisc.edu/botany_401/lecture/03Lecture.html