What is Glycogenolysis?
- Glycogenolysis is the metabolic process through which glycogen, a stored form of glucose in animals, is broken down to release glucose. Glycogen is a polysaccharide made up of glucose molecules, primarily stored in the liver and skeletal muscles. This process is crucial for maintaining energy balance in the body, especially during fasting or periods of increased energy demand.
- In glycogenolysis, glycogen undergoes enzymatic breakdown, starting with the release of glucose-1-phosphate by the action of glycogen phosphorylase. This enzyme removes glucose units sequentially from the non-reducing ends of glycogen. The glucose-1-phosphate is then converted into glucose-6-phosphate by the enzyme phosphoglucomutase. In liver cells, glucose-6-phosphate is further processed by glucose-6-phosphatase to release free glucose into the bloodstream, maintaining blood sugar levels.
- The liver plays a central role in glycogenolysis by ensuring a steady glucose supply during fasting or when blood glucose levels drop. In contrast, glycogen breakdown in skeletal muscles primarily supports local energy needs during physical activity, as muscle cells lack glucose-6-phosphatase and cannot release free glucose into the bloodstream.
- This process is tightly regulated by hormonal signals and energy requirements. Hormones like glucagon and adrenaline stimulate glycogenolysis by activating signaling pathways that enhance glycogen phosphorylase activity. Conversely, insulin inhibits the process, promoting glycogen synthesis instead.
- Glycogenolysis is vital for cellular energy production. Glucose derived from this pathway enters glycolysis, where it is metabolized to generate adenosine triphosphate (ATP), the molecule that powers numerous cellular activities. The process also supports critical metabolic functions by providing substrates for biosynthetic pathways, such as the synthesis of glycoproteins.
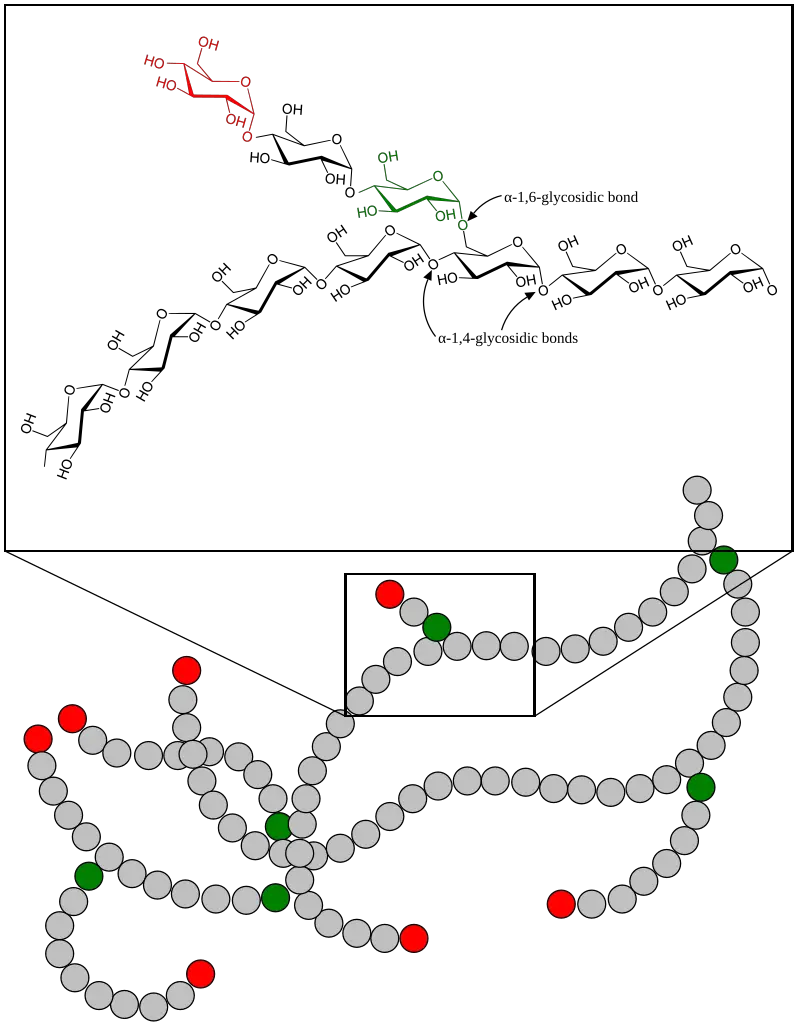
Glycogenolysis Definition
Glycogenolysis is the enzymatic process of breaking down glycogen, a stored polysaccharide, into glucose-1-phosphate and glucose to provide energy or maintain blood sugar levels during fasting or increased energy demand.
Glycogenolysis Location
Glycogenolysis occurs in the cytoplasm of cells, primarily in the liver and skeletal muscles, with minimal activity in adipose tissue. These locations are essential for meeting the body’s energy demands and maintaining glucose homeostasis.
In the liver, glycogenolysis serves as a key mechanism for regulating blood glucose levels. When blood sugar levels drop, the liver breaks down its glycogen stores and releases glucose into the bloodstream, ensuring a continuous supply for tissues and organs, particularly the brain.
In skeletal muscles, glycogenolysis provides energy for contraction and physical activity. Muscle cells utilize their glycogen reserves to produce glucose-6-phosphate, which enters glycolysis to generate adenosine triphosphate (ATP) for immediate energy. Unlike the liver, muscle cells do not release glucose into the bloodstream, as they lack the enzyme glucose-6-phosphatase.
While adipose tissue has some capacity for glycogen storage and breakdown, its role in glycogenolysis is negligible compared to the liver and muscles. These differences underscore the specialized functions of each tissue in energy metabolism and glucose regulation.
Glycogenolysis Enzymes
Glycogenolysis is the biochemical process that breaks down glycogen into glucose to meet the body’s energy demands. Several enzymes are key players in this process, each performing a specific function to ensure efficient glycogen breakdown.
- Glycogen Phosphorylase:
- This enzyme is at the core of glycogenolysis.
- It cleaves alpha-1,4 glycosidic bonds in glycogen, releasing glucose-1-phosphate.
- This reaction occurs in the cytosol, making glucose-1-phosphate available for energy production.
- Debranching Enzymes:
- These enzymes help break down the branching alpha-1,6 glycosidic bonds within glycogen.
- The debranching process is crucial because it allows glycogen phosphorylase to continue breaking down the glycogen molecule.
- Two primary enzymes involved in this process are:
- Alpha-1,4-glucantransferase
- Alpha-1,6-glucosidase
- Phosphoglucomutase:
- After glycogen phosphorylase releases glucose-1-phosphate, phosphoglucomutase converts it into glucose-6-phosphate.
- In the liver, kidneys, and intestines, glucose-6-phosphate can be converted into free glucose for the bloodstream.
- In muscle cells, however, glucose-6-phosphate is used for local energy production as muscle cells lack glucose-6-phosphatase.
- Phosphorylase Kinase:
- This enzyme activates glycogen phosphorylase by phosphorylating it.
- The activation of phosphorylase kinase occurs as part of a hormonal signaling cascade initiated by glucagon and epinephrine.
- Phosphorylase kinase works in muscle cells by binding to adenyl cyclase and cAMP, which activates it and facilitates the conversion of phosphorylase b to the active form, phosphorylase a.
Glycogenolysis Enzymes Summary Table
| Enzyme | Function | Location | Key Action |
|---|---|---|---|
| Glycogen Phosphorylase | Cleaves alpha-1,4 glycosidic bonds in glycogen, releasing glucose-1-phosphate. | Cytosol | Releases glucose-1-phosphate from glycogen for energy production. |
| Debranching Enzymes | Breaks down alpha-1,6 glycosidic bonds in glycogen. | Cytosol | Allows glycogen phosphorylase to continue breaking down glycogen. Includes alpha-1,4-glucantransferase and alpha-1,6-glucosidase. |
| Phosphoglucomutase | Converts glucose-1-phosphate to glucose-6-phosphate. | Liver, kidney, intestines | Converts glucose-1-phosphate to glucose-6-phosphate; in liver, used for glucose release. In muscle, used for energy. |
| Phosphorylase Kinase | Activates glycogen phosphorylase by phosphorylation. | Cytosol | Activated by hormones like glucagon and epinephrine to trigger glycogen breakdown. |
Glycogenolysis Steps
Glycogenolysis, the breakdown of glycogen into glucose, follows a detailed series of enzymatic actions. This process, crucial for maintaining energy supply, occurs in the liver and muscles. Below are the key steps involved in glycogenolysis:
- Step 1: Activation of Glycogen Phosphorylase
- The process begins with the activation of glycogen phosphorylase, an enzyme responsible for breaking down glycogen.
- In the liver, glucagon triggers the activation, while in muscles, epinephrine also plays a significant role.
- These hormones bind to their respective receptors, initiating a signaling cascade that activates protein kinase A (PKA).
- PKA then activates phosphorylase kinase, which in turn activates glycogen phosphorylase, setting the process in motion.
- Reaction:
Hormone + Receptor → cAMP pathway → PKA activation → Phosphorylase kinase activation → Glycogen phosphorylase activation
- Step 2: Glycogen Breakdown by Glycogen Phosphorylase
- Activated glycogen phosphorylase acts on the glycogen molecule by cleaving the α-1,4-glycosidic bonds between glucose residues.
- This results in the release of glucose-1-phosphate from the non-reducing ends of the glycogen chain.
- This step essentially shortens the glycogen chain, preparing it for further processing.
- Reaction:
Glycogen (n residues) + Pi → Glycogen (n-1 residues) + Glucose-1-phosphate
- Step 3: Action of the Branching Enzyme
- As the glycogen molecule is broken down, the branching enzyme (also known as amylo-(1,4→1,6)-transglycosylase) comes into play.
- This enzyme transfers a block of 3 to 4 glucose units from the branch of the glycogen molecule to a different branch point, forming a new non-reducing end. This allows glycogen phosphorylase to continue breaking down glycogen.
- Chemical reaction:
Glycogen + Debranching enzyme → (Glucose)_n-1-m + (Glucose)_m+1 → (Glucose)_n-m + Glucose
- Step 4: Action of the Debranching Enzyme
- Once the glycogen chain reaches a point where it is about four glucose units from a branch point, the debranching enzyme (glycogen-debranching enzyme) acts.
- It hydrolyzes the α-1,6-glycosidic bond at the branch point, releasing a free glucose molecule.
- The remaining glycogen can continue to be processed by glycogen phosphorylase.
- Step 5: Conversion of Glucose-1-Phosphate
- The released glucose-1-phosphate is then converted into glucose-6-phosphate by the enzyme phosphoglucomutase.
- This form of glucose is commonly funneled into glycolysis to produce ATP, providing energy to the cell.
Throughout these steps, glycogen is broken down into glucose-1-phosphate, which is eventually used to generate ATP, especially when the body is in need of energy. The process is tightly regulated to ensure that glucose is available when needed but not overproduced.
Glycogenolysis Regulations
Glycogenolysis is a tightly controlled process, essential for maintaining the body’s energy balance. Its regulation ensures glucose availability during periods of fasting or physical exertion while preventing excessive breakdown when energy is not needed.
- Hormonal Control
- Glucagon
When blood glucose levels drop, the pancreas releases glucagon. This hormone targets the liver, triggering glycogenolysis by activating specific signaling pathways. Glucagon binds to receptors on liver cells, leading to the activation of phosphorylase kinase (PhK), which then phosphorylates glycogen phosphorylase (GP), converting it to its active form (GPa). - Epinephrine
During stress or “fight-or-flight” responses, epinephrine is released, initiating a similar process. It activates glycogenolysis through pathways that mirror glucagon’s effects, ensuring a quick energy release when required. - Insulin
Insulin plays the opposite role. When glucose levels are high, it inhibits glycogenolysis, promoting glycogen synthesis instead. Insulin works by reducing the activity of glycogen breakdown enzymes, ensuring glucose is stored rather than released.
- Glucagon
- Allosteric Regulation
Glycogen phosphorylase (GP), the key enzyme in glycogenolysis, is regulated by allosteric factors.- Activators
AMP and inorganic phosphate (Pi) enhance GP activity, signaling the need for more glucose. These activators ensure glycogen breakdown is ramped up during energy-demanding conditions. - Inhibitors
When glucose or glucose-6-phosphate levels rise, they inhibit GP activity, signaling sufficient energy availability. This prevents unnecessary glycogen breakdown when the cell has adequate fuel.
- Activators
- Phosphorylation Mechanism
The activation of glycogenolysis is heavily dependent on phosphorylation.- Phosphorylation of GP
Hormones like glucagon and epinephrine trigger the phosphorylation of glycogen phosphorylase. Phosphorylase kinase (PhK) adds a phosphate group to GP at serine 14, switching it from the inactive (GPb) to the active (GPa) form. - Switch Mechanism
Phosphorylation acts as a switch, turning the enzyme on or off depending on the hormonal signals and the cell’s needs. This mechanism allows the body to respond quickly to changes in energy demands.
- Phosphorylation of GP
Clinical Significance of Glycogenolysis
Glycogenolysis is critical for regulating blood glucose levels, and disruptions in this pathway can contribute to several clinical conditions. Here are the key areas where glycogenolysis is clinically significant:
- Glycogen Storage Diseases (GSDs)
Several genetic disorders affect glycogenolysis by impairing enzymes involved in the breakdown of glycogen. These include:- McArdle Disease (GSD V)
- Cause: Deficiency of muscle glycogen phosphorylase.
- Symptoms: Exercise intolerance, muscle cramps, and risk of rhabdomyolysis. Myoglobin released from damaged muscles can lead to acute renal failure.
- Cori Disease (GSD III)
- Cause: Deficiency in the debranching enzyme.
- Symptoms: Inability to fully degrade glycogen, leading to its accumulation in the liver and muscles. Symptoms include hepatomegaly, delayed growth, and muscle weakness.
- Andersen Disease (GSD IV)
- Cause: Deficiency of the branching enzyme.
- Symptoms: Abnormal glycogen structures that cannot be used effectively, leading to liver cirrhosis and potential heart failure due to tissue scarring at a young age.
- Von Gierke Disease (GSD I)
- Cause: Deficiency of glucose-6-phosphatase.
- Symptoms: Impaired gluconeogenesis and glycogenolysis, causing severe fasting hypoglycemia, lactic acidosis, hyperuricemia, and hyperlipidemia. A swollen abdomen due to hepatomegaly is also common.
- Liver Phosphorylase Deficiency (GSD VI)
- Cause: Deficiency of liver phosphorylase.
- Symptoms: Milder form of GSD with hypoglycemia and hepatomegaly.
- Phosphorylase Kinase Deficiency (GSD IX)
- Cause: Deficiency in phosphorylase kinase, which activates glycogen phosphorylase.
- Symptoms: Muscle weakness and exercise intolerance similar to McArdle disease, with added liver-related symptoms depending on severity.
- McArdle Disease (GSD V)
- Diabetes
In diabetes, glycogenolysis can become dysregulated, especially when insulin production is absent or insufficient (as in type 1 diabetes). This leads to improper glucose synthesis, causing elevated blood glucose levels (hyperglycemia). - Exercise and Sports Performance
Glycogenolysis plays a role in athletic performance by providing glucose for muscle energy during exercise. Athletes often adjust their training and diet to optimize glycogen reserves and delay glycogen depletion, maximizing endurance and performance.
Functions of Glycogenolysis
Glycogenolysis is a critical metabolic process responsible for breaking down glycogen into glucose. This function supports various physiological needs, particularly during periods of energy demand or glucose scarcity.
- Glucose Supply for Energy
Glycogenolysis provides glucose as an energy source during fasting or physical exertion.
Glycogen stored in the liver is broken down to maintain blood glucose levels, ensuring energy availability for essential functions like brain activity and red blood cell metabolism. - Maintenance of Blood Glucose Levels
By breaking down glycogen in the liver, glycogenolysis prevents hypoglycemia during fasting or prolonged activity.
The liver releases glucose into the bloodstream, stabilizing glucose levels and maintaining metabolic balance. - Energy Production in Muscles
In muscle cells, glycogenolysis supplies glucose-6-phosphate, fueling glycolysis and producing ATP.
ATP generated from this process supports muscle contractions and other energy-intensive cellular activities. - Immediate Energy Source During Exercise
Glycogen stored in muscles is directly converted into glucose during high-intensity exercise.
This process bypasses the liver and provides energy locally, allowing muscles to maintain performance even when oxygen is limited. - Glucose Redistribution for Other Tissues
Liver glycogenolysis releases glucose into the bloodstream, making it accessible to organs like the brain and red blood cells.
This redistribution ensures a consistent energy supply for tissues that rely exclusively on glucose. - Energy Storage and Utilization
Glycogen serves as a readily mobilizable form of glucose storage in the liver and muscles.
Excess glucose from food is stored as glycogen, which can be broken down when blood glucose levels drop or energy demands increase. - Support for Cellular Functions
The ATP generated from glycogen-derived glucose supports various cellular processes, including ion transport, biosynthesis, and maintaining homeostasis.
This energy also facilitates active transport mechanisms and molecular synthesis essential for cellular operation.
Difference Between Glycolysis and Glycogenolysis
Glycolysis and glycogenolysis are both critical pathways in glucose metabolism, but they serve different functions in energy production and take place in different contexts.
Here’s how these processes differ:
| Feature | Glycolysis | Glycogenolysis |
|---|---|---|
| Definition | Breakdown of glucose into pyruvate, ATP, and NADH. | Breakdown of glycogen into glucose. |
| Location | Occurs in the cytoplasm of all cells. | Primarily occurs in liver and muscle cells. |
| Starting Material | Begins with glucose. | Begins with glycogen, a polymer of glucose. |
| End Products | Produces two molecules of pyruvate, ATP, and NADH. | Produces glucose-1-phosphate and free glucose. |
| Enzymes Involved | Key enzymes include hexokinase, phosphofructokinase, and pyruvate kinase. | Key enzymes include glycogen phosphorylase and phosphorylase kinase. |
| Energy Yield | Generates a net gain of 2 ATP molecules per glucose molecule. | Does not directly produce ATP; glucose enters glycolysis for ATP production. |
| Regulation | Regulated by energy levels (ATP/ADP ratio) and hormonal signals (insulin/glucagon). | Regulated by hormonal signals (glucagon/epinephrine) and allosteric effectors (AMP, glucose). |
These distinctions reflect the unique roles each pathway plays in cellular energy production, with glycolysis being the direct breakdown of glucose for ATP and NADH generation, while glycogenolysis is the process that mobilizes stored glucose from glycogen for energy production when needed.
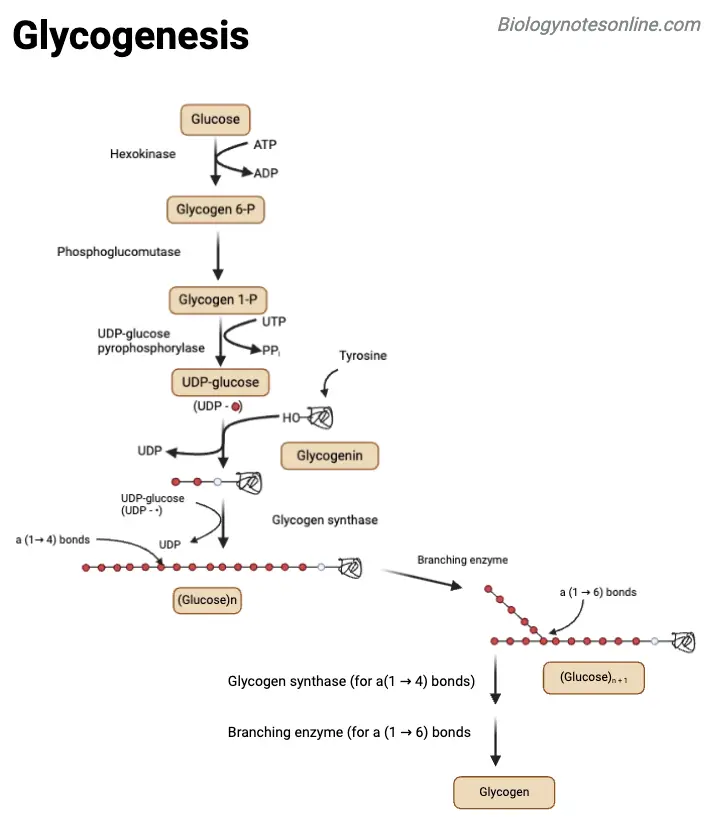
Difference Between Glycogenesis and Glycogenosis
Glycogenesis and glycogenosis are both vital processes in glycogen metabolism but serve opposite functions in energy management within the body. Here’s a comparison:
| Feature | Glycogenesis | Glycogenosis |
|---|---|---|
| Definition | The process of synthesizing glycogen from glucose. | Often used to describe glycogenolysis, the breakdown of glycogen into glucose. |
| Process Type | An anabolic process (building up glycogen). | A catabolic process (breaking down glycogen). |
| Function | Stores excess glucose as glycogen for future energy needs. | Releases glucose from glycogen for immediate energy use. |
| Location | Primarily in the liver and muscle cells. | Mainly in the liver and muscle cells as well. |
| Key Enzymes | Includes glycogen synthase and branching enzyme. | Involves glycogen phosphorylase and debranching enzyme. |
| Regulation | Activated by insulin when blood glucose levels are high. | Stimulated by glucagon and epinephrine when energy is needed. |
These two processes balance energy storage and energy mobilization. Glycogenesis builds up glycogen from glucose when there’s an excess of energy, while glycogenosis breaks down glycogen to provide glucose when the body requires immediate energy.
- Salih, Karzan & Sabir, Dana & Abdoul, Hayman. (2022). Glycolysis Regulation to Maintain Blood Glucose Homeostasis. Kurdistan Journal of Applied Research. 7. 114-124. 10.24017/Scince.2022.1.10.
- Dashty Rahmatabady, Monireh. (2013). A quick look at biochemistry: Carbohydrate metabolism. Clinical biochemistry. 46. 10.1016/j.clinbiochem.2013.04.027.
- Stone WL, Basit H, Adil A. Glycogen Storage Disease. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459277/
- Dashty, M. (2013). A quick look at biochemistry: Carbohydrate metabolism. Clinical Biochemistry, 46(15), 1339–1352. doi:10.1016/j.clinbiochem.2013.04.027
- Paredes-Flores MA, Rahimi N, Mohiuddin SS. Biochemistry, Glycogenolysis. 2024 Jan 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 32119304.
- Nadeau OW, Fontes JD, Carlson GM. The regulation of glycogenolysis in the brain. J Biol Chem. 2018 May 11;293(19):7099-7107. doi: 10.1074/jbc.R117.803023. Epub 2018 Feb 26. PMID: 29483194; PMCID: PMC5950003.
- https://www.genetex.com/Research/Overview/metabolism/Glycogenesis_Glycogenolysis?srsltid=AfmBOorKZTVD91r-dnCd_PuqsJp_VcrguolZSIvDDA0zDGMkxqmd6cnw
- https://en.wikipedia.org/wiki/Glycogenolysis
- https://www.pw.live/exams/neet/glycogenolysis/
- https://byjus.com/neet/glycogenolysis/
- https://www.sciencefacts.net/glycogenesis.html
- https://www.simplepharmanotes.com/2022/07/glycogenesis.html
- https://microbenotes.com/glycogenesis/