Gluconic Acid is the 16 stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid. It is an inorganic chemical. It occurs naturally in honey, plants, and wine. The first carbon of glucose is oxidised to form gluconic acid, which has antibacterial and chelating effects. Please include the chemical composition and further details.
Properties Of Gluconic Acid
| Chemical formula | C6H12O7 |
| Molecular weight | 196.155 g/mol |
| Melting point | 131 °C |
| Chemical names | Dextronic acid and D-Gluconic acid |
| solubility | Soluble in water |
| Acidity (pKa) | 3.86 |
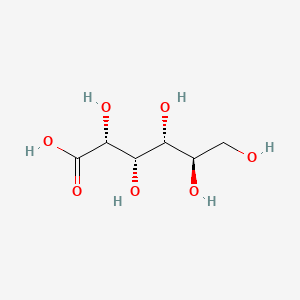
Gluconic Acid Structural Formula
The structure of gluconic acid consists of a six-carbon chain and five hydroxyl groups arranged in the normal open-chain format of glucose, followed by a carboxylic acid group. In the presence of cyclic ester glucono delta-lactone, gluconic acid exists in a condition of equilibrium in water. The structural formula of Gluconic Acid is depicted in the image below.
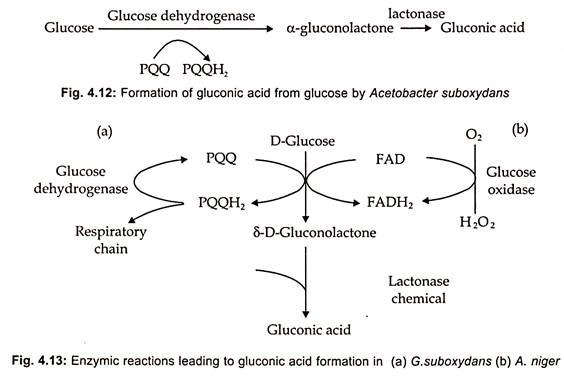
Production of Gluconic Acid
- There are various methods for producing gluconic acid, including chemical, electrochemical, biochemical, and bioelectrochemical processes.
- There are numerous oxidising agents available, however the process looks to be more expensive and inefficient than fermentation techniques. Even though the conversion is a straightforward one-step procedure, the chemical technique is not preferred.
- Thus, fermentation has been one of the most effective and prevalent methods for producing gluconic acid.
- The approach involving the fungus A. niger is one of the most extensively employed microbial fermentation procedures. However, the G. oxydans method has also gained considerable relevance.
- Regardless of whether fungi or bacteria are used, the value lies in the final product, such as sodium gluconate or calcium gluconate, etc.
- As the reaction produces an acidic product, it must be neutralised by the addition of neutralising agents; otherwise, the acidity deactivates the glucose oxidase, halting the synthesis of gluconic acid.
- The fermentation conditions for the formation of calcium gluconate and sodium gluconate differ in numerous ways, including glucose concentration (initial and final) and pH regulation. The inclusion of calcium carbonate slurry allows for pH regulation during the manufacture of calcium gluconate.
- Also noteworthy is the solubility of calcium gluconate in water (4 percent at 30 degrees Celsius). At glucose concentrations above 15%, supersaturation occurs, and if the concentration surpasses the limit, calcium salt precipitates on the mycelia and prevents oxygen transmission.
- The neutralising agent must also be sterilised separately from the glucose solution in order to avoid the Lobry de Bruyn-van Ekenstein reaction, which affects the structure of glucose and causes a 30% decrease in yield.
- In contrast, the sodium gluconate method is vastly preferred, as glucose concentrations of up to 350 g/L can be employed without any issues.
- The pH is regulated by the addition of NaOH solution. At 30 °C, sodium gluconate is 39.6% soluble in water.
1. Fungal Fermentation
- Fungal fermentation utilises a submerged fermentation process. Aspergillus niger is a microorganism that is utilised.
- As inoculum, either sporulated culture or spores germinated in a seed tank are employed. Each approach has its own merits. For instance, the use of spore inoculum eliminates the expense of installation and operation of the seed tank, whilst the use of germinated spores reduces the number of working cycles for the main fermenter.
- Glucose is utilised as a solution, crystalline glucose, or syrup made from starch or crude starchy material that has been processed with amylase and amyloglucosidase.
- Through strong agitation with the aid of a turbido mixture or cavitator, it is required to maintain the highest concentration of dissolved oxygen in solution.
- In the production of calcium or sodium gluconate, the optimal temperature and pH are 28-30°C and 6.5, respectively, with strong agitation and aeration.
a. Recovery and Harvest
- After fermentation, the fungal mycelium is extracted via filtration, and the separated mycelium is utilised in the glucose recovery process. On the other hand, calcium or sodium gluconate is recovered from the filtrate.
b. Production of Calcium Gluconate
The following procedures are used to recover calcium gluconate:
- The filtrate is heated with Ca(OH)2 in excess.
- The final product is filtered and decolored with activated carbon.
- The chemical is crystallised by cooling it below 20 degrees Celsius and then seeding it with calcium gluconate crystals.
- The leftover mother liquor (about 10 to 15 percent) is used to produce a second crop of calcium gluconate through heating, carbon filtering, and chilling.
c. Production of Sodium Gluconate
Recovery of sodium gluconate is extremely straightforward and comprises of the processes listed below.
- The concentration of the filtrate is between 42 and 45 percent.
- The addition of sodium hydroxide crystallises the sodium gluconate and maintains a pH of 7.5.
- The resulting sodium gluconate is then drum-dried.
d. Production of Pure Gluconic Acid
It is also possible to get pure gluconic acid by the following technique involving calcium gluconate:
- Calcium is precipitated when sulfuric acid is added. Thus produced calcium sulphate is separated via filtration.
- Activated carbon is used to decolorize the filtrate.
- The acid solution is concentrated to a 50% acid concentration. Thus, a combination of gluconic acid and lactones is produced.
- The concentrate is then subjected to temperatures between 0°C and 30°C. Pure gluconic acid constitutes the crystals that form at this temperature.
- When the concentrate is heated between 30 and 70 degrees Celsius, lactone crystals separate.
- The separation of Y-lactone crystals occurs when the concentrate is heated to 70°C or higher.
2. Bacterial Fermentation
- The fermentation process is submerged. Acetobacter suboxydans or Gluconobacter suboxydans are the bacteria used.
- By repeated sub-culturing, a pure culture of the bacterium that is used in the fermentative synthesis of gluconoic acid is grown. This pure culture is produced either as calcium gluconate or sodium gluconate.
- In the fermentation, glucose serves as a substrate. Due to the low solubility of calcium gluconate (4 g liter-1 at 30°C), 13-15% glucose can be added as a substrate for producing calcium gluconate.
- If glucose concentrations above 13%, greater amounts of calcium gluconate are produced, which makes purification difficult. Calcium gluconate crystallises spontaneously as a result.
- For the production of sodium gluconate, a glucose concentration of 28-30% can be employed.
- Fermentation is conducted at 28-30°C, pH 4.5-6.5, with a high aeration rate of 1-1.5 vol/vol/min.
- The gluconic acid output can be significantly enhanced (90-95%) by increasing the oxygen’s solubility, which is accomplished by increasing the system’s pressure.
Uses of Gluconic Acid
- Gluconic acid is used to produce metal, leather, and food.
- In numerous detergents, sodium gluconate is utilised as a sequestering agent.
- Calcium gluconate is used in medicine.
- As a baking powder and an ingredient, gluconolactone is utilised.