What is Germplasm conservation?
- Germplasm conservation plays a critical role in maintaining the genetic diversity of plant species. This practice, deeply rooted in agricultural history, began when humans first recognized the need to domesticate wild plant species about 10,000 years ago. Early agricultural societies, especially in regions like India and China as early as 700 BC, learned to preserve seeds or vegetative parts of plants to ensure future crops. This foundational practice helped in introducing genetic variability through natural hybridization and mutation, laying the groundwork for modern agriculture.
- Plant genetic resources (PGRs) refer to the entire pool of genetic material in a given species, including traits drawn from wild ancestors, cultivated varieties, and landraces. Germplasm, on the other hand, is the actual genetic material passed down through generations. The diversity of this genetic material, which includes both cultivated and wild relatives, is essential for food security and agricultural sustainability. Crop wild relatives (CWRs), for instance, are especially valuable for introducing new traits, such as disease resistance or drought tolerance, into cultivated plants.
- Despite the importance of preserving germplasm, several challenges exist. While gene banks store over 7.5 million accessions globally, representing crops and their wild relatives, there are still many locally significant and underutilized species that remain unprotected. Additionally, there is a gap between the vast genetic collections available in these banks and their actual use in breeding programs. Breeders often rely on a narrow genetic pool, which can limit the potential for crop improvement.
- Germplasm conservation is more than just preserving seeds in storage facilities; it is about safeguarding the genetic heritage of plants that have evolved over centuries. Human activities, particularly selective breeding, have led to the loss of certain genetic traits, making it even more important to conserve the remaining diversity. This genetic material is crucial not only for food production but also for other applications, such as forestry, horticulture, and medicinal plants.
History of Germplasm Conservation
Germplasm conservation has evolved through significant scientific and international efforts to safeguard the genetic diversity of crop plants. Below is a detailed historical account highlighting key milestones:
- Alphonse de Candolle’s Contribution (1882): Alphonse de Candolle, a botanist, made the first major attempt to trace the origins of crop plants. His work, titled Origin of Cultivated Plants, was initially published in 1882 and reprinted in 1959. It laid the foundation for future exploration into the origins of agricultural species.
- N.I. Vavilov’s Centers of Origin (1926): Russian geneticist and plant breeder N.I. Vavilov proposed the concept of ‘centers of origin’ for crop plants. He identified eight geographic regions where crop plants exhibited maximum genetic diversity. In 1951, Vavilov expanded his findings and introduced the distinction between primary centers (where crops originated and held maximum diversity) and secondary centers (regions where crops evolved independently after migrating from their origins).
- Zhukovsky’s Mega and Micro Gene Centers (1965): Zhukovsky modified Vavilov’s centers into eight mega gene centers and four micro gene centers for crop wild relatives. This classification broadened the understanding of where genetic diversity is concentrated globally.
- Gene Pools Concept (1971): Harlan and De Wet (1971) developed the gene pool concept, categorizing genetic variation based on the ease of cross-breeding species:
- Primary Gene Pool (GP1): Includes cultivated and wild races that can naturally cross and recombine, allowing gene transfer through standard breeding.
- Secondary Gene Pool (GP2): Consists of species that have partial crossability barriers with GP1, often producing sterile hybrids due to abnormal chromosome pairing.
- Tertiary Gene Pool (GP3): More distantly related species, which produce lethal or sterile hybrids with GP1, but may still be useful through specialized tissue culture techniques.
- Gene Ocean (GP4): Introduced through advancements in genetic engineering, this concept allows gene transfer between different kingdoms using recombinant DNA technology.
- International Union for Conservation of Nature (1948): The establishment of the IUCN marked the first global collaboration between governments and civil society organizations to protect nature. It indirectly set the stage for germplasm conservation by raising awareness about biodiversity protection.
- FAO’s Role (1960s): The Food and Agriculture Organization (FAO) held its first international technical meeting on plant exploration and introduction in 1961, which emphasized locating, classifying, preserving, and coordinating plant genetic resources globally. In 1968, FAO’s Crop Ecology and Genetic Resources Unit further strengthened international cooperation on managing plant genetic resources (PGRs).
- International Agricultural Research Centers (1960s-1970s): The Rockefeller Foundation and World Bank established International Agricultural Research Centers (IARCs) in many developing countries. These centers developed their own germplasm collections, laying the groundwork for global crop conservation.
- United Nations Conference on Human Environment (1972): The UN recognized the threat of genetic resource erosion and recommended conserving habitats rich in genetic diversity during this conference.
- International Board of Plant Genetic Resources (1974): The IBPGR was established at FAO headquarters in Rome to promote a global network for collecting, conserving, and utilizing plant germplasm.
- Convention on Biological Diversity (1992): This landmark international agreement aimed at the conservation and sustainable use of biological diversity, including plant genetic resources. It also promoted the equitable sharing of benefits from using these resources.
- FAO Global Plan of Action for PGRFA (1996): This plan aimed to ensure the conservation of plant genetic resources for food and agriculture (PGRFA), promote sustainable use, reduce hunger, and support agricultural development.
- International Treaty on Plant Genetic Resources for Food and Agriculture (2001): Signed by 136 countries, this treaty emphasized the conservation and sustainable use of PGRs for food and agriculture, aligning with the objectives of the CBD.
- Global Strategy for Plant Conservation (Early 2000s): The strategy aimed to promote the sustainable future of plant life by preserving at least 75% of endangered plant species in ex situ collections by 2020.
- Nagoya Protocol (2010): This protocol under the CBD encouraged fair and equitable sharing of benefits from the use of genetic resources.
Methods of Germplasm Storage and Conservation
There are two methods for Germplasm Storage and Conservation
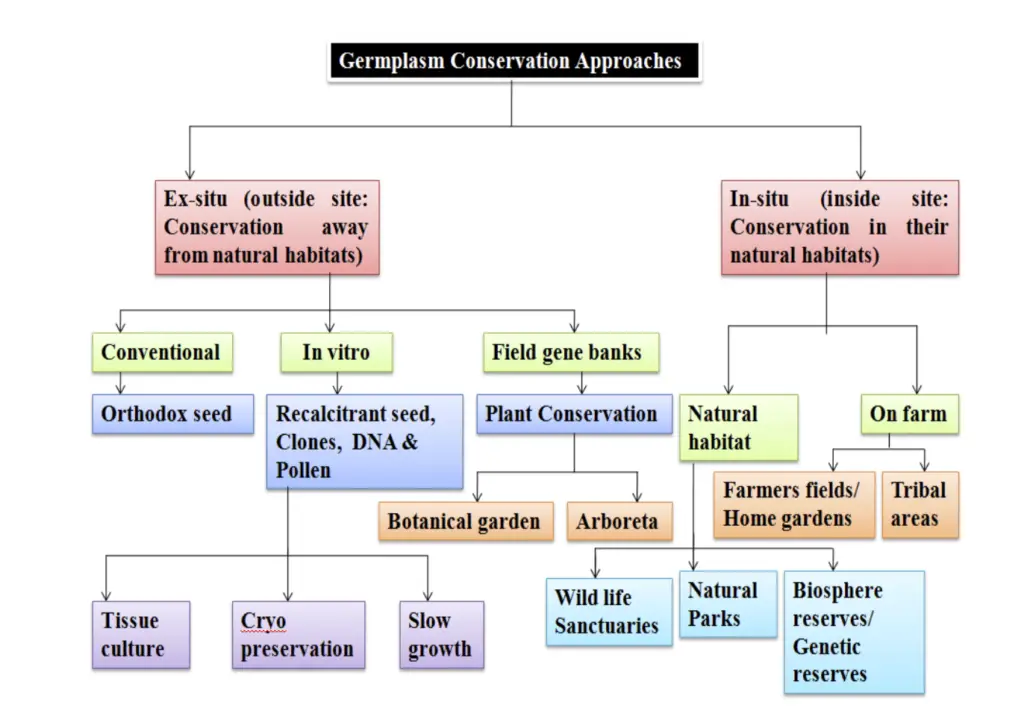
A. Ex Situ Conservation
Ex situ conservation of germplasm refers to the preservation of plant genetic resources outside their natural habitats. This approach is essential for safeguarding biodiversity and ensuring the availability of genetic materials for agricultural and ecological research. The following points outline the key aspects of ex situ conservation, including methods, types of seeds, and storage considerations.
- Overview of Ex Situ Conservation:
- Ex situ management is a straightforward and cost-effective strategy that involves regular viability testing and timely recovery of germplasm, depending on the specific crop and its reproductive biology.
- The collection of wild species in seed banks is projected to play a vital role in preserving and restoring biodiversity. Efficient management of these collections is crucial for obtaining viable seeds for optimal utilization.
- Role of the Global Crop Diversity Trust (GCDT):
- The GCDT significantly contributes to improving ex situ conservation techniques and managing global crop diversity.
- Its efforts focus on enhancing the methodologies for preserving genetic materials, thereby ensuring the long-term availability of plant resources.
- Types of Seeds:
- Seeds stored in seed banks are classified into two primary categories based on their storage requirements: orthodox seeds and recalcitrant seeds.
- Orthodox Seeds:
- These seeds can tolerate drying (approximately 5% relative humidity) and freezing at very low temperatures while remaining viable.
- The majority of plant species fall into this category, allowing for long-term preservation of their seeds.
- Examples of orthodox seeds include citrus, guava, capsicum, cashew, and most grains and legumes.
- Recalcitrant Seeds:
- In contrast, recalcitrant seeds cannot withstand drying and freezing; they lose viability significantly when moisture content drops below 30–50%.
- These seeds typically require careful handling and can be stored at temperatures between 0–10 °C for a brief period (1 to 5 years) while maintaining viability.
- Examples include several tropical trees and fruits such as pineapples, cocoa, coffee, oil palm, mango, and jackfruit.
- Viability Factors:
- Viability of seeds can be influenced by moisture content and storage temperature.
- Generally, for every decrease of 1% in seed moisture content, the lifespan of the seed doubles, applicable when the moisture content is between 5-14%.
- Similarly, a decrease of 5 °C in storage temperature can also double the seed’s lifespan, applicable within the range of 0 °C to 50 °C.
- Handling and Storage Considerations:
- The method of handling and storing crop species varies depending on whether the stored material is seed or clone, and whether the seed is orthodox or recalcitrant.
- Conservation techniques requiring specialized methods, such as tissue culture, cold storage, liquid nitrogen storage, and cryopreservation, tend to be more costly than the preservation of orthodox seeds.
- Additionally, desiccation and storage of embryos represent another ex situ conservation method; however, this approach typically utilizes somatic embryos and shoot tips.
1. In Vitro Conservation
In Vitro Conservation is a vital technique in plant preservation, particularly for species that cannot be maintained through traditional seed storage methods. It encompasses various strategies to conserve plant genetic resources, especially those that exhibit unique challenges in germplasm preservation. This approach relies on plant tissue culture, which allows for the cultivation of plant cells or tissues in a controlled environment. The following points outline the key aspects and methods of in vitro conservation:
- Historical Context: The concept of in vitro conservation emerged in the mid-1970s as a response to the limitations of conventional seed preservation techniques. This method is especially crucial for species like potato and banana, which either do not produce viable seeds or have recalcitrant seeds that are challenging to store.
- Fundamentals of In Vitro Conservation: The in vitro conservation process involves aseptically separating a cell or tissue from the donor plant and cultivating it on a synthetic medium within a controlled environment. This protocol often begins by reviewing existing methodologies for similar taxa, ensuring the selected technique aligns with the physiological and biochemical characteristics of the species in question.
- Protection Against Viral Infections: To safeguard conserved species from viral infections, techniques such as meristem cultivation or cryotherapy can be employed. These methods focus on preserving the health and viability of plant tissues during conservation.
- Methods of In Vitro Conservation of Germplasm: Several strategies have been identified as effective in in vitro conservation, including:
- In Vitro Active Gene Bank (IVAG): This technique involves maintaining plant tissues in a slow-growth state, allowing for short- to medium-term conservation. It is widely used by national and international research institutions, although it is limited in capacity for extensive collections.
- Cryopreservation: This method utilizes extreme low temperatures (e.g., liquid nitrogen at −196 °C) to preserve plant cells and tissues in a frozen state. It is essential for the long-term preservation of various plant tissues, including meristems, eggs, and protoplasts. Cryopreservation aims to mitigate cellular damage caused by ice crystal formation, employing two main strategies:
- Vitrification: This process involves using cryoprotective agents to transform cellular water into a non-crystalline solid.
- Encapsulation: This technique involves embedding tissues in alginate gel to form artificial seeds, which are then dehydrated for preservation.
- Slow-Growing Cultures: This alternative to cryopreservation is cost-effective and minimizes contamination risks. Cultures can remain viable for extended periods, but they may be susceptible to genetic instability and infection.
- DNA Storage: Establishing a DNA storage facility serves as a supplementary method to traditional conservation, providing genomic material that aids in understanding species origin and diversity.
- Cold Storage: This technique involves storing germplasm at non-freezing temperatures (1–9 °C), allowing for continued growth and reducing cryogenic damage. Cold storage is both economical and effective, yielding higher survival rates for germplasm.
- Low-Pressure and Low-Oxygen Storage: Low-pressure storage reduces atmospheric pressure around plant materials, slowing growth and extending shelf life. Similarly, low-oxygen storage decreases oxygen concentration, further limiting growth and preserving germplasm for both short and long-term applications.
- Challenges of Recalcitrant Seeds: Certain tropical and subtropical plant species produce seeds that are recalcitrant and thus unsuitable for long-term storage due to their physiological immaturity and high moisture content. These challenges necessitate the adoption of modern in vitro techniques, such as freezing and cold storage, to ensure successful preservation.
- Advantages of In Vitro Conservation: This method offers numerous benefits, including adaptability and stability while minimizing contamination risks. However, contamination is influenced by various factors such as tissue age, position, complexity, and environmental conditions.
2. Ex Situ Conservation in Field Gene Banks, Botanic Gardens, and Arboreta
Ex Situ Conservation in Field Gene Banks, Botanic Gardens, and Arboreta plays a crucial role in the preservation of plant genetic diversity. These institutions are designed to conserve plant species outside their natural habitats, offering safe environments for the maintenance and study of various plant materials. Each of these conservation methods has unique characteristics and functions, contributing significantly to global biodiversity efforts.
- Field Gene Banks:
- Traditionally, field gene banks have focused on conserving recalcitrant and vegetative species, such as fruit trees, tubers, and plantation crops.
- Germplasm is cultivated in field nurseries at varying elevations depending on the specific needs of the species being preserved.
- This method allows for the maintenance of whole plant collections, which can be accessed for research and breeding purposes.
- Botanic Gardens:
- Botanic gardens serve multiple purposes, including educational exhibitions, economic exploitation, and scientific research.
- Globally, there are approximately 1,700 botanical gardens, housing over 3.2 million live accessions representing around 100,000 different species.
- A significant portion of the plant species in these gardens, estimated at 10 to 15%, are considered at risk in their natural habitats.
- Approximately half of these at-risk species are actively conserved through various policies and practices.
- The first botanical garden was established in Pisa, Italy, during the 17th century, paving the way for numerous others that have contributed to studies on plant taxonomy and horticultural advancements.
- Arboreta:
- Arboreta are specialized gardens dedicated to the cultivation of trees, shrubs, and other woody plants.
- These institutions often focus on the conservation and display of species that are particularly important for ecological restoration, education, and landscape management.
- Arboreta can provide valuable resources for understanding tree genetics and species adaptation in various environmental conditions.
- Global Accessions and Germplasm Representation:
- Various gene banks around the world hold substantial collections of crop and wild species. For instance:
- Wheat (Triticum): 856,168 accessions at CIMMYT in Mexico, representing 13% of the world’s germplasm.
- Rice (Oryza): 773,948 accessions at IRRI in the Philippines, accounting for 14% of global germplasm.
- Maize (Zea): 327,932 accessions at CIMMYT, contributing 8% of the world’s genetic resources.
- Bean (Phaseolus): 261,963 accessions at CIAT in Colombia, representing 14%.
- Apple (Malus): 59,922 accessions in the USA, constituting 12% of global diversity.
- Palm (Elaeis): 21,103 accessions at INFRA in the Democratic Republic of Congo, which encompasses 84%.
- Medicago: 91,922 accessions at AMGRC in Australia, representing 30%.
- Cacao (Theobroma): 12,373 accessions at ICGT in Trinidad, comprising 19% of global germplasm.
- Various gene banks around the world hold substantial collections of crop and wild species. For instance:
B. In Situ Conservation
In Situ Conservation refers to the preservation of plant genetic resources for food and agriculture (PGRFA) within their natural habitats. This approach maintains genetic diversity in the wild, whether in natural ecosystems or traditional agricultural settings. In situ conservation emphasizes the continuity of species while allowing for natural evolution and adaptation, contributing significantly to biodiversity.
- Genetic Diversity in Natural Habitats:
- The genetic diversity of PGRFA is preserved in environments such as forests, grasslands, and agricultural landscapes.
- Nature reserves, national parks, and gene sanctuaries serve to protect ecosystems and wildlife species, rather than focusing solely on individual PGRFAs.
- A key advantage of in situ conservation is its ability to facilitate species continuity and the emergence of new recombinant types, which may enhance genetic diversity over time.
- Challenges of In Situ Conservation:
- In situ conservation faces several disadvantages, including a lack of effective protection without managed surveillance.
- Environmental contaminants can threaten the integrity of germplasm, potentially degrading its viability.
- Additionally, the high costs associated with maintaining a vast number of genotypes may limit the practicality of in situ approaches.
- The longevity and immediate usability of conserved germplasm are also uncertain, posing further challenges to conservation efforts.
- Turkey is recognized for its significant advancements in developing effective strategies for in situ conservation of genetic diversity.
- Natural Reserves or Genetic Reserves:
- The primary goal of establishing natural reserves is to enhance genetic diversity while minimizing the number of reserves needed.
- Effective conservation requires comprehensive data on the genetic diversity, population composition, breeding mechanisms, habitat needs, and geographical distribution of target taxa.
- The process of establishing a natural reserve involves several steps:
- Planning the Reserve: Determine the objectives and necessary components of the reserve.
- Site Assessment: Evaluate the site’s ecological, socioeconomic, and political factors.
- Reserve Design: Formulate the physical layout and management strategies for the reserve.
- Taxon Sustainability Assessment: Ensure the viability of the species within the reserve.
- Management Plan Formulation: Create guidelines for the ongoing management and monitoring of the reserve.
- Implementation: Begin executing the management plan and establish the reserve physically.
- Traditional or Professional Use: Consider how the reserve will be utilized in terms of research or community practices.
- Linkage to Ex Situ Conservation: Integrate efforts with complementary research and educational programs to enhance conservation outcomes.
- An illustrative example of such a conservation initiative is the Ammiad experiment in Israel, which studies the naturally occurring diversity of the wild T. turgidum species.
- On-Farm and Home Garden Conservation:
- Farmers play a vital role in in situ conservation through the maintenance of common crop varieties within traditional farming systems.
- These systems often include the cultivation of landraces, where farmers harvest crops and reserve portions of the seed for subsequent planting.
- In this context, farmers act as custodians of genetic diversity, either intentionally or inadvertently preserving germplasm.
- Although this method has benefits, it carries risks, as farmers may transition from cultivating landraces to modern cultivars, leading to potential loss of valuable genetic resources for future generations.
Applications of Germplasm Storage
Germplasm storage, particularly through cryopreservation, is an essential technique for conserving plant genetic resources. It allows for the long-term storage of plant materials without the need for frequent maintenance, ensuring the viability of valuable genetic material over extended periods. The applications of germplasm storage are vast and critical for both agricultural and conservation efforts, addressing a wide range of biological, ecological, and commercial needs.
- No Need for Sub-culturing: One of the primary benefits of cryopreservation for germplasm storage is that it eliminates the need for continuous sub-culturing. Stock cultures can be maintained in a frozen state without requiring frequent transfers or care, which reduces the risk of contamination and genetic drift over time.
- Long-term Conservation of Cell Cultures: Cryopreservation is an ideal method for the long-term storage of cell cultures, particularly those that produce valuable secondary metabolites. These compounds, such as medicines and biologically active substances, can be preserved in a stable state for extended periods, ensuring their availability for future use.
- Storage of Disease-free Plant Materials: Germplasm storage allows for the preservation of pathogen-free plant materials. These disease-free materials can be stored and propagated as needed, ensuring that healthy plants can be grown without the risk of disease transmission.
- Maintenance of Recalcitrant Seeds: Recalcitrant seeds, which do not survive traditional drying and freezing methods, can be preserved using cryopreservation techniques. This allows for the long-term maintenance of these seeds, which are often vital for the survival of certain plant species.
- Conservation of Somaclonal and Gametoclonal Variations: In tissue cultures, somaclonal and gametoclonal variations represent important genetic diversity. Cryopreservation helps in conserving these variations, which can be valuable for breeding programs and for maintaining genetic diversity in plant populations.
- Conservation of Endangered Species: Germplasm storage plays a critical role in the conservation of endangered plant species. By preserving plant materials from species at risk of extinction, cryopreservation helps ensure their survival for future generations.
- Conservation of Pollen: Pollen can be cryopreserved to extend its longevity, which is particularly useful for breeding programs. By storing pollen, the genetic material can be used for crossbreeding or hybridization at a later time, even beyond the typical lifespan of the pollen.
- Storage of Rare Germplasm: Rare germplasm, including material developed through somatic hybridization and other genetic manipulations, can be stored using cryopreservation. This allows for the preservation of unique genetic resources that might otherwise be lost.
- Selection of Cold-resistant Mutant Cell Lines: Germplasm storage provides a reliable method for selecting cold-resistant mutant cell lines. These cell lines can be used to develop frost-resistant plants, which are crucial for agricultural production in cold climates.
- Establishment of Germplasm Banks: Germplasm banks serve as repositories for storing plant genetic material, facilitating the exchange of information at national and international levels. These banks ensure that valuable plant materials are preserved and made accessible for research, breeding, and conservation efforts worldwide.
- Veerala, Dr & Kumar, Rahul & Dhaliwal, Inderpreet & Kaushik, Prashant. (2021). Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review. Sustainability. 13. 6743. 10.3390/su13126743.
- https://www.slideshare.net/slideshow/germ-plasm-conservation-80347611/80347611
- https://www.researchgate.net/figure/Wheat-germplasm-conservation-strategies_fig3_357912410