| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Bifidobacteriales |
| Family: | Bifidobacteriaceae |
| Genus: | Gardnerella |
| Species: | G. vaginalis |
What is Gardnerella Vaginalis?
Gardnerella vaginalis is a micro-aerophilic coccobacillus that is a constituent of the vaginal microbiota. This bacterium, while present in the vaginal flora, does not inherently lead to bacterial vaginosis unless its populations become predominant. Contrary to some beliefs, Gardnerella vaginalis might not solely be a consequence of sexual transmission but could be an integral part of the normal vaginal flora.
- The vaginal microbiota is predominantly composed of Lactobacillus species, notably Lactobacillus crispari, Lactobacillus gasseri, and Lactobacillus jensenii.
- However, other bacteria, such as Actinomycetes, Bifidobacterium, and Gardnerella vaginalis, are also present in healthy females. It is noteworthy that Gardnerella can be present in pre-pubescent females, indicating that the vagina is not a sterile environment post-puberty.
- The prevalence of this bacterium in the vaginal microbiota can fluctuate based on factors like immunity, sexual activity, antibiotic usage, and physiological changes, especially around menopause.
- A pivotal role in maintaining vaginal health is played by the Lactobacillus species. These bacteria enhance the vaginal acidity levels to a range of 3.8 to 4.5 by secreting hydrogen peroxide and lactic acid. This acidic milieu inhibits the proliferation of potentially pathogenic bacteria.
- Furthermore, Lactobacillus bacteria obstruct many harmful bacteria from adhering to the vaginal epithelium, thereby impeding their growth or colonization. A balanced vaginal microbiota, primarily constituted of Lactobacillus, acts as a protective shield against infections.
- However, certain factors, including hormonal shifts, sexual activity, douching, dietary habits, illness, and specific medications, can disrupt this balance. A decline in Lactobacillus levels can pave the way for other bacteria to colonize the vagina, altering its pH and potentially leading to infections. Bacterial vaginosis, predominantly caused by Gardnerella, is the most frequent vaginal infection.
- From a morphological perspective, Gardnerella vaginalis is characterized as a Gram-variable-staining facultative anaerobic bacterium. These organisms are non-spore-forming, nonmotile coccobacilli with a diameter ranging from 1.0 to 1.5 μm.
- Historically classified as Haemophilus vaginalis and later as Corynebacterium vaginalis, G. vaginalis thrives on specific media like chocolate agar and HBT agar. A selective medium tailored for G. vaginalis is the colistin-oxolinic acid blood agar.
- It is essential to understand that Gardnerella is not strictly categorized as a sexually transmitted bacterium. While it can be transferred between women during sexual activities or to men, leading to colonization in the male urethra, it often remains asymptomatic.
- In conclusion, Gardnerella vaginalis is a complex bacterium that can coexist harmoniously within the vaginal flora but can also be the primary agent in bacterial vaginosis when its populations become dominant.
Habitat of Gardnerella vaginalis
Gardnerella vaginalis is a bacterium that occupies specific niches within the human body. Its presence is not limited to pathological conditions but can also be a part of the normal flora.
- Vaginal Flora: Gardnerella vaginalis is detected in the vaginal flora of approximately 40% of females. It is essential to note that in these instances, the bacterium is present in minimal amounts. Moreover, it has been isolated from the vaginas of females of reproductive age and the male urethra, especially in partners of women diagnosed with bacterial vaginosis.
- Urine: This bacterium can be identified in urine samples, indicating its potential association with the urinary tract.
- Blood and Wounds: Although its presence in blood and wounds is rare, Gardnerella vaginalis can occasionally be found in these sites, suggesting its potential to invade other body sites under specific conditions.
- Anorectal Flora: Gardnerella vaginalis is not exclusive to the female reproductive system. It has been isolated from the anorectal flora of healthy adults, irrespective of gender, and even in children. This highlights its widespread distribution within the human body.
- Optimal Growth Conditions: The bacterium exhibits optimal growth at a pH range between 6 and 7. This slightly acidic to neutral pH range is conducive for its proliferation.
- Clinical Implications: Beyond its presence as a part of the normal flora, Gardnerella vaginalis has been implicated in certain medical conditions. It has been associated with cervical cancer and infections of the urinary tract. Furthermore, it is frequently found in the vaginas of patients who exhibit no symptoms, underscoring the bacterium’s potential to exist asymptomatically.
In summary, Gardnerella vaginalis is a versatile bacterium with a diverse habitat within the human body. While it can exist as a harmless component of the normal flora, its association with specific medical conditions emphasizes the need for a comprehensive understanding of its biology and behavior.
Morphology of Gardnerella vaginalis
Gardnerella vaginalis is a bacterium with distinct morphological characteristics that set it apart from other bacterial species. A comprehensive examination of its morphology reveals the following features:
- Size: Gardnerella vaginalis is a relatively small bacterium, with dimensions approximately 1.28 x 0.3-0.6µm.
- Spore Formation: The bacterium does not undergo sporulation, meaning it does not produce spores as a part of its life cycle.
- Motility: Gardnerella vaginalis is non-motile, indicating it does not possess structures like flagella to facilitate movement.
- Capsulation and Endospore Formation: The bacterium neither forms a capsule around its cell wall nor produces endospores, both of which are mechanisms some bacteria use for protection.
- Filamentous Nature: Gardnerella vaginalis is non-filamentous, meaning it does not exhibit a thread-like structure.
- Gram Staining: While primarily characterized as Gram-negative, Gardnerella vaginalis can sometimes exhibit Gram-variable characteristics. This means that it may not consistently stain as either Gram-positive or Gram-negative, making it unique in this aspect.
- Shape and Structure: The bacterium is pleomorphic, suggesting it can assume various shapes. It often appears as rods or coccobacilli. Additionally, metachromatic granules, which can take up stains of different colors, are present within the bacterial cells.
- Clue Cells: One of the hallmark features of Gardnerella vaginalis is the presence of “clue cells.” These are vaginal epithelial cells that have a stippled appearance due to the adhering bacteria.
- Acid-Fast Property: Gardnerella vaginalis is non-acid fast, meaning it does not retain the primary stain when subjected to an acid-fast staining procedure.
- Oxygen Requirements: The bacterium is facultative anaerobic, indicating it can grow in both the presence and absence of oxygen.
- Cell Wall: The cell wall of Gardnerella vaginalis is laminated, providing it with a structured and layered appearance.
In conclusion, the morphology of Gardnerella vaginalis is characterized by a combination of unique and common bacterial features. Understanding these characteristics is crucial for its identification and study in microbiological research and clinical diagnostics.
Genome of Gardnerella vaginalis
The genome of Gardnerella vaginalis provides insights into its biology, physiology, and evolutionary history. A meticulous examination of its genomic attributes reveals the following characteristics:
- Genomic Structure: The genome of Gardnerella vaginalis is composed of circular DNA, a common feature in many bacteria, which provides a continuous loop of genetic information.
- Genome Size: The size of the Gardnerella vaginalis genome ranges between 1.67 Mb (megabases) to 1.72 Mb. This size indicates the amount of genetic information encoded within the bacterium, which in turn dictates its biological functions and capabilities.
- G + C Content: The guanine (G) and cytosine (C) content of the Gardnerella vaginalis genome is estimated to be between 42 to 44%. The G + C content can offer insights into various aspects of the bacterium, including its evolutionary relationships, stability of the DNA, and potential gene regulation mechanisms.
- Plasmids: As of the current understanding, no plasmids have been identified in Gardnerella vaginalis. Plasmids are small, circular DNA fragments that exist outside the main chromosome in bacteria and often carry genes that can provide the bacterium with advantageous traits. The absence of plasmids in Gardnerella vaginalis suggests that its genetic adaptability might be primarily reliant on its chromosomal DNA.
In summary, the genome of Gardnerella vaginalis is a compact and structured entity that encodes the essential genetic information for the bacterium’s survival, growth, and function. Understanding its genomic attributes is pivotal for in-depth studies on its biology, pathogenicity, and potential therapeutic interventions.
Bacteriological characteristics of Gardnerella vaginalis
Gardnerella vaginalis, a bacterium associated with the vaginal flora, possesses distinct bacteriological attributes that aid in its identification and understanding. A comprehensive analysis of its bacteriological characteristics reveals the following:
- Gram Staining: Gardnerella vaginalis is categorized as Gram-variable. This means that, during the Gram staining procedure, it may not consistently stain as either Gram-positive or Gram-negative. Instead, it can appear as bacilli (rod-shaped) or coccobacilli (short rods with a shape intermediate between cocci and bacilli).
- Enzymatic Activities:
- Catalase Test: Gardnerella vaginalis is catalase-negative. This indicates that the bacterium lacks the enzyme catalase, which breaks down hydrogen peroxide into water and oxygen.
- Oxidase Test: The bacterium is also oxidase-negative, signifying the absence of the cytochrome c oxidase enzyme in its electron transport chain.
- Sodium Hippurate Hydrolysis: A distinguishing feature of Gardnerella vaginalis is its ability to hydrolyze sodium hippurate, resulting in a positive test outcome.
- Carbohydrate Metabolism: Gardnerella vaginalis demonstrates acid production from specific carbohydrates. It can metabolize glucose and maltose consistently to produce acid. However, its ability to produce acid from sucrose is variable, implying that not all strains of the bacterium might exhibit this trait.
- Culture Conditions:
- Culture Medium: For optimal growth and isolation, Gardnerella vaginalis is preferably cultured on specialized media. Vaginal agar (V agar) and Human Blood Tween (HBT) agar are the preferred media for its cultivation.
- Growth Conditions: The bacterium requires anaerobic conditions for optimal growth. Culturing is typically carried out for a duration of 48 hours to ensure adequate growth and isolation.
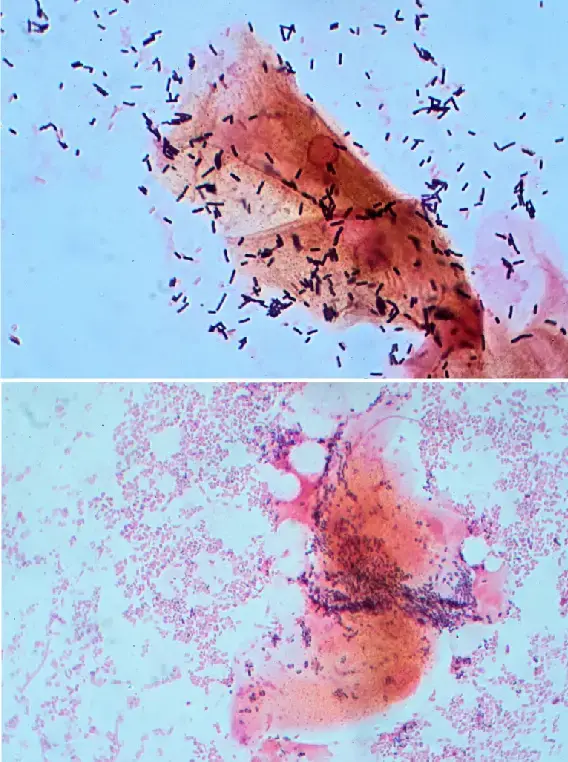
How Does Gardnerella Cause Bacterial Vaginosis?
Gardnerella vaginalis is a micro-aerophilic coccobacillus, which means it thrives in environments with extremely low oxygen concentrations. While it is often termed anaerobic, it does necessitate minimal oxygen levels for proliferation. Ambient oxygen levels are detrimental to Gardnerella, and its non-motile nature means it remains stationary in low-oxygen niches. When there’s an imbalance in the protective vaginal bacteria, Gardnerella can colonize, often leading to the formation of a biofilm.
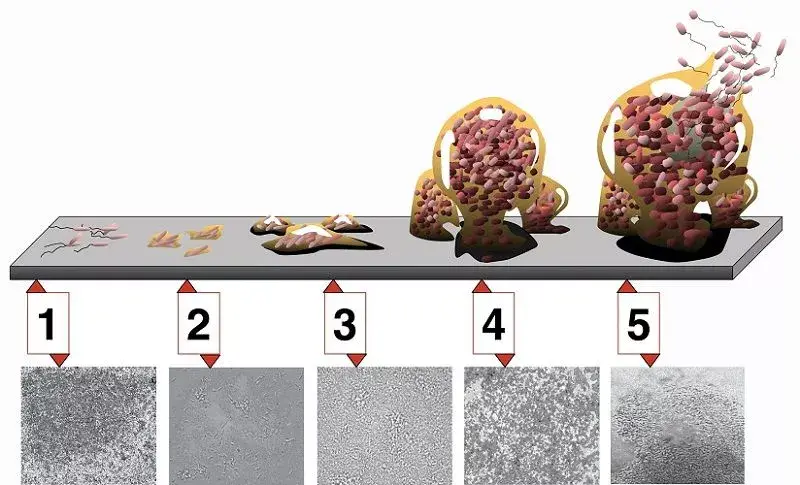
Understanding Biofilms: Biofilms are structured communities of microorganisms that adhere to surfaces and are enveloped in a self-produced polymeric matrix. Individual pathogenic bacterial cells cannot instigate infections; infections arise when these cells multiply and colonize specific tissues. Biofilms can form wherever microorganisms accumulate, from physiological systems to external environments.
In the context of the vaginal microbiota, the biofilm formation process involves several stages:
- Initial Reversible Attachment: Gardnerella vaginalis first adheres to the vaginal wall. At this juncture, interventions can effectively eliminate them.
- Irreversible Attachment: The bacteria utilize surface proteins to strengthen their adherence to the vaginal epithelial cells. Once firmly attached, they progress to form a microcolony.
- Microcolony Formation: The bacteria multiply, establishing a community. They then produce extracellular polymeric substances (EPS), which constitute up to 90% of the biofilm and provide a protective layer. The EPS also captures nutrients, benefiting the resident bacteria.
- Maturation: The biofilm environment, with its nutrient-rich and optimal temperature conditions, facilitates the rapid proliferation of Gardnerella.
Gardnerella Vaginalis in Bacterial Vaginosis: A salient characteristic of G. vaginalis is its capacity to form synergistic associations with other pathogenic bacteria. Initial colonization by Gardnerella often paves the way for the invasion of other detrimental bacterial species. Consequently, G. vaginalis is frequently identified as the primary causative agent of bacterial vaginosis (BV).
Despite its non-motile nature, G. vaginalis can still disperse from its biofilm. Factors such as changes in nutrient availability or temperature can trigger a subset of cells to detach from the biofilm and migrate to other regions, ensuring the survival of the bacterial population. This adaptability and resilience make infections caused by G. vaginalis challenging to treat.
In summary, the ability of Gardnerella vaginalis to form biofilms and collaborate with other pathogenic bacteria plays a pivotal role in its association with bacterial vaginosis. Understanding this mechanism is crucial for developing effective therapeutic strategies against BV.
Gardnerella Vaginalis Symptoms
Gardnerella vaginalis is a bacterium that plays a significant role in the onset of bacterial vaginosis (BV). While it is a component of the vaginal flora, its overgrowth, in conjunction with other bacteria, can lead to BV. Intriguingly, over half of the women with BV may remain asymptomatic. However, when symptoms manifest, they can include:
- Vaginal Discharge: Women may observe a discharge that can vary in color from gray to white or green. This discharge often has a pronounced and unpleasant odor.
- Odor Post-Coitus: A distinct fishy smell may be noticeable after sexual intercourse.
- Vaginal Itching: This is a common symptom, causing discomfort to the affected individual.
- Urination Discomfort: A burning sensation might be experienced during urination.
- Post-Coital Bleeding: Some women may experience vaginal bleeding after sexual activity.
While Gardnerella vaginalis is primarily associated with the genital tract, it can, in rare instances, lead to severe infections in other areas. Cases of sepsis, wound infections, and infections in men have been documented, albeit infrequently.
From a bacteriological perspective, G. vaginalis is a facultatively anaerobic, Gram-variable rod. It is involved in BV due to disruptions in the standard vaginal microflora. The vaginal environment is typically acidic, maintained by the resident facultative anaerobic Lactobacillus population. When anaerobic bacteria overtake the native vaginal bacteria, the ecosystem’s balance is disrupted, necessitating antibiotic intervention to restore the natural flora. It’s crucial to note that G. vaginalis is not the sole cause of BV but rather an indicator of the altered microbial ecology characterized by the overgrowth of multiple bacterial species.
G. vaginalis can be identified in various samples, including blood, urine, and the pharynx. Even though it is a predominant bacterium in BV, it can also be found in women who display no infection symptoms. Morphologically, it possesses a Gram-positive cell wall but can appear Gram-variable due to its thin cell wall. Microscopically, it is associated with “clue cells,” which are epithelial cells densely covered in bacteria.
Furthermore, G. vaginalis produces a specific toxin, vaginolysin, which exclusively affects human cells. The presence of protease and sialidase enzyme activities is also frequently associated with G. vaginalis.
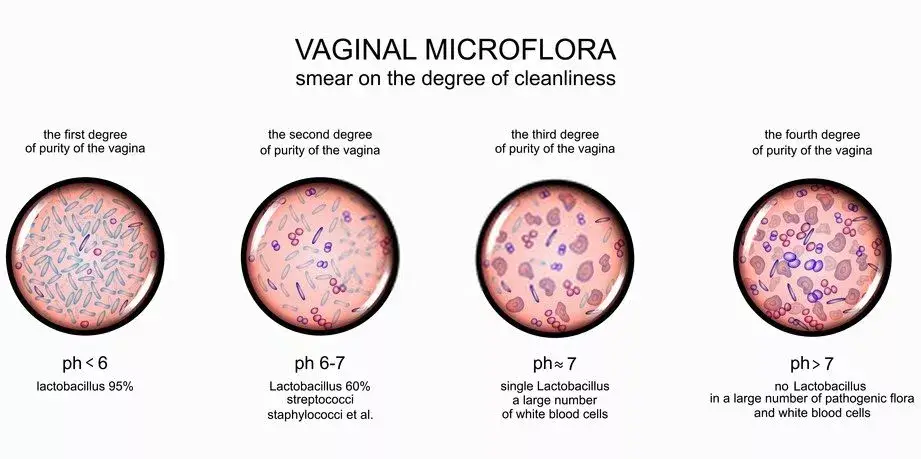
Gardnerella Vaginalis Diagnosis
The accurate diagnosis of Gardnerella Vaginalis, a bacterium implicated in bacterial vaginosis (BV), is crucial for effective treatment and management. Several diagnostic techniques have been developed to identify the presence of this bacterium, each with its unique methodology and sensitivity. Here’s an overview of the primary diagnostic methods:
- OSOM BV Blue Assay: This is a chromogenic point-of-care test designed to measure sialidase levels in vaginal fluids. Sialidases are enzymes produced by bacteria, including Gardnerella and Bacteroides species. The presence of elevated sialidase levels can indicate an ongoing BV infection, making the OSOM BV Blue Assay a valuable diagnostic tool.
- FemExam Cards: These diagnostic cards serve a dual purpose. Firstly, they can detect the pH of vaginal fluid and the presence of trimethylamine, both of which can indicate BV. Secondly, they can measure the proline iminopeptidase activity specific to G. Vaginalis. Proline iminopeptidase is an enzyme produced by Gardnerella Vaginalis, and its detection can confirm the presence of this bacterium.
- Nucleic Acid Amplification Tests (NAATs): Among the most sensitive diagnostic techniques, NAATs, such as polymerase chain reaction (PCR), can detect even minute quantities of an organism in a sample. This high sensitivity allows for the accurate identification of Gardnerella Vaginalis, even when present in low numbers. The ability of NAATs to detect as few as a single organism provides a comprehensive insight into the status of Gardnerella Vaginalis and associated BV.
In conclusion, the diagnosis of Gardnerella Vaginalis and bacterial vaginosis requires a combination of clinical observation and laboratory testing. The aforementioned diagnostic techniques, each with its unique advantages, offer clinicians a range of tools to accurately identify and manage infections caused by Gardnerella Vaginalis.
Gardnerella Vaginalis Treatment
Bacterial vaginosis (BV), often associated with the overgrowth of Gardnerella Vaginalis, necessitates effective therapeutic interventions. The treatment strategies for BV, particularly those caused by G. Vaginalis, encompass a range of antibiotics and alternative therapeutic agents. Here’s a detailed overview:
- Antibiotic Treatment:
- Metronidazole: This antibiotic is frequently prescribed for BV. It operates by targeting G. Vaginalis, thereby mitigating the symptoms and infection.
- Clindamycin: Research, such as the study published in the Journal of Clinical Microbiology, has highlighted the efficacy of clindamycin against G. Vaginalis. In vitro studies have demonstrated that clindamycin can inhibit the growth of G. Vaginalis at concentrations as minimal as 0.25 µg/mL.
- Alternative Treatment Options:
- Boric Acid: With the rising concern of antibiotic resistance, there’s a pressing need for alternative treatments. Boric acid has emerged as a promising candidate in this regard. Studies, like the one by Sobel et al. in 2009, have shown boric acid’s effectiveness against BV. It achieves this by eliminating BV-specific biofilms, thereby enhancing the eradication of G. Vaginalis and other associated bacterial pathogens. This results in a decreased recurrence of symptomatic BV. While the exact mechanism of action of boric acid remains to be fully elucidated, its potential effects on bacterial enzymes, biofilm formation, and bacterial cell wall permeability render it a potent treatment option, especially for recurrent BV.
In conclusion, while antibiotics like metronidazole and clindamycin remain the mainstay of BV treatment, the emergence of antibiotic resistance underscores the importance of alternative therapeutic agents like boric acid. A comprehensive understanding of these treatment modalities ensures optimal management of infections caused by Gardnerella Vaginalis.
Quiz
Which of the following best describes the morphology of Gardnerella vaginalis?
a) Motile, spore-forming bacillus
b) Non-motile, spore-forming coccus
c) Non-motile, non-sporing coccobacillus
d) Motile, non-sporing coccus
Which diagnostic technique measures sialidase levels in vaginal fluids for detecting Gardnerella vaginalis?
a) FemExam Cards
b) PCR
c) OSOM BV Blue Assay
d) Blood culture
Gardnerella vaginalis is primarily associated with which of the following conditions?
a) Urinary tract infection
b) Bacterial vaginosis
c) Yeast infection
d) Pelvic inflammatory disease
Which antibiotic is commonly used to treat bacterial vaginosis caused by Gardnerella vaginalis?
a) Penicillin
b) Ciprofloxacin
c) Metronidazole
d) Rifampicin
Which alternative treatment has shown efficacy against bacterial vaginosis by removing BV-specific biofilms?
a) Ascorbic acid
b) Boric acid
c) Citric acid
d) Hyaluronic acid
Which enzyme activity is frequently associated with Gardnerella vaginalis?
a) Catalase
b) Oxidase
c) Sialidase
d) Lysozyme
Gardnerella vaginalis is considered a:
a) Strict aerobe
b) Strict anaerobe
c) Facultative anaerobe
d) Microaerophile
Which of the following is NOT a symptom of bacterial vaginosis caused by Gardnerella vaginalis?
a) Fishy odor after sex
b) Gray vaginal discharge
c) Fever and chills
d) Vaginal itching
Which stage of biofilm formation involves the use of surface proteins by Gardnerella vaginalis to adhere to vaginal epithelial cells?
a) Initial reversible attachment
b) Irreversible attachment
c) Microcolony formation
d) Maturation
Which diagnostic tool measures the proline iminopeptidase activity specific to G. Vaginalis?
a) PCR
b) Blood culture
c) FemExam Cards
d) OSOM BV Blue Assay
FAQ
What is Gardnerella vaginalis?
Gardnerella vaginalis is a bacterium that is commonly found in the vaginal flora of many women. It is one of the primary bacteria associated with bacterial vaginosis (BV).
Is Gardnerella vaginalis a sexually transmitted infection (STI)?
No, Gardnerella vaginalis is not considered an STI. However, its overgrowth can lead to bacterial vaginosis, which can be influenced by sexual activity.
What are the symptoms of an infection caused by Gardnerella vaginalis?
Symptoms can include a fishy-smelling vaginal odor, grayish-white vaginal discharge, vaginal itching, and burning during urination.
How is Gardnerella vaginalis diagnosed?
Diagnosis can be made through various techniques such as the OSOM BV Blue Assay, FemExam Cards, and nucleic acid amplification tests (NAATs) like PCR.
What treatments are available for bacterial vaginosis caused by Gardnerella vaginalis?
Common treatments include antibiotics like metronidazole or clindamycin. Alternative treatments like boric acid have also shown efficacy against BV.
Can men be carriers of Gardnerella vaginalis?
Yes, while Gardnerella vaginalis primarily affects women, men can be carriers of the bacterium in their urethra without showing symptoms.
Is bacterial vaginosis caused only by Gardnerella vaginalis?
No, while Gardnerella vaginalis is a primary bacterium associated with BV, the condition results from an imbalance of the vaginal flora, involving multiple bacterial species.
How can one prevent an overgrowth of Gardnerella vaginalis?
Maintaining a healthy vaginal pH, practicing safe sex, and avoiding douching can help prevent an imbalance in the vaginal flora and potential overgrowth of Gardnerella vaginalis.
Can bacterial vaginosis caused by Gardnerella vaginalis recur after treatment?
Yes, even after successful treatment, BV can recur. It’s essential to follow the prescribed treatment regimen and consult a healthcare professional if symptoms return.
Is Gardnerella vaginalis the same as a yeast infection?
No, Gardnerella vaginalis is associated with bacterial vaginosis, while yeast infections are caused by an overgrowth of fungi, primarily Candida species. The symptoms and treatments for the two conditions are different.
References
- Kairys N, Garg M. Gardnerella. [Updated 2022 Aug 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459350/
- Catlin BW. Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin Microbiol Rev. 1992 Jul;5(3):213-37. doi: 10.1128/CMR.5.3.213. PMID: 1498765; PMCID: PMC358241.
- Kairys N, Garg M. Gardnerella. [Updated 2022 Aug 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459350/
- Front. Cell. Infect. Microbiol., 24 April 2020 Sec. Clinical Microbiology Volume 10 – 2020 | https://doi.org/10.3389/fcimb.2020.00168
- https://www.sciencedirect.com/topics/medicine-and-dentistry/gardnerella-vaginalis
- https://microbiologie-clinique.com/gardnerella-vaginalis-cause-of-bacterial-vaginosis.html
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.