What is Flame Photometer?
- An analytical instrument called a flame photometer measures the concentration of certain metal ions in an aqueous solution via the light emitted by the atoms excited in a flame. Bowling Barnes, David Richardson, John Berry and Robert Hood developed it in the 1980s to measure low levels of sodium and potassium in samples. The International Union of Pure and Applied Chemistry (IUPAC) also refers to this technique as flame atomic emission spectrometry (FAES).
- A flame photometer works on the principle of atom excitation. Metal ions contained in a sample will become excited when introduced into a flame and will emit light at certain wavelengths. Because the emitted light is the same color as the particular type of metal ion, this gives a few advantages, ability to know which ions are in the sample by the emitted luminescence and their concentration since the amount of light emitted is directly proportional to the concentration of the ion in the sample.
- In analytical chemistry, flame photometry also belongs to atomic absorption spectroscopy and is used to detect and quantify sodium, potassium, lithium, calcium and cesium. Because of its simplicity and convenience in the analysis of metal ions at a variety of solutions, this method is mostly employed, yielding important information for purposes in scientific and industrial sectors.
Principle of Flame photometer
The flame photometer is based on the principle that alkali and alkaline earth metal atoms are excited in the flame and that the light emitted when the excited atoms revert to the ground state can be measured. When you introduce a sample containing metal compounds into a flame, the very high temperature of the flame causes them to dissociate into free atoms. The thermal energy is absorbed by some of these atoms, which then become excited.
The atoms are excited and unstable in the process and dissipate some energy as they go back down to their ground state. This energy is released in light, with radiation emitted at a specific wavelength unique to each element. For example, sodium burns with a 589 nm yellow light and potassium burns at 766 nm in violet. Likewise, barium produces a pastel green glow (554 nm), calcium produces a bright orange glow (622 nm), and lithium produces a bright red glow (670 nm).
Since the light emitted intensity is proportional to the number of atoms returning to the ground state, those numbers are also directly related to the concentration of metal ions in the sample. Flame photometer measures ion concentration accurately due to this relation which makes it suitable model in analytical chemistry.
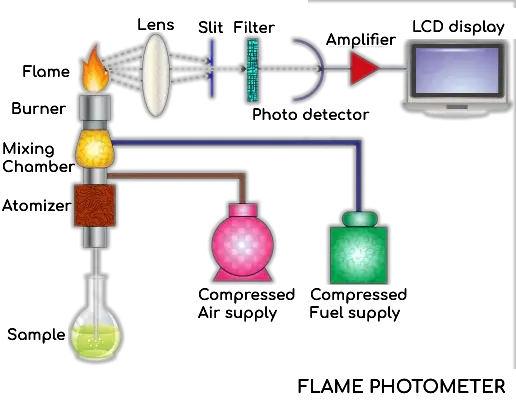
Parts of a Flame Photometer
The main components of a flame photometer include: All parts are vital for the correct functioning of the measuring plan.
- Source of Flame
- The main component in this system is the burner which generates the flame. The one thing that keeps the burner metric from changing is temperature, which needs to have as stable as possible conditions for reliable measurements.
- The flame temperature is dependent upon the fuel-oxidant mixture used:
- Natural gas-Air: 1700°C
- Propane-Air: 1800°C
- Hydrogen-Air: 2000°C
- Hydrogen-Oxygen: 2650°C
- Acetylene-Air: 2300°C
- Acetylene-Oxygen: 3200°C
- Acetylene-Nitrous oxide:2700°C
- Cyanogen-Oxygen: 4800°C
- The flame temperature is dependent upon the fuel-oxidant mixture used:
- These are directly related to the thickening of the sample indicating the excitation of metal atoms.
- The main component in this system is the burner which generates the flame. The one thing that keeps the burner metric from changing is temperature, which needs to have as stable as possible conditions for reliable measurements.
- Nebuliser – The nebuliser generates the sample solution into an aerosol and injects it into the flame at a rate that can be controlled to be constant. This amounts to the procession of the solution being homogeneously incorporated into the a flame, ensuring accurate readings.
- Optical System – The system is composed of a convex mirror and a convex lens. The excited atoms emit light which the convex mirror focuses toward the lens.
- Colour Filters – Once it has passed the mirror and lens, the light goes through a series of simple colour filters. Wavelength-selective filters separate the light wavelengths corresponding to the metal ions measured and prevent emissions from other elements.
- Photo-detector – It puts the photo-detector (responsible for measuring the intensity of emitted radiation) It turns the light into an electrical signal, which can then be processed. This signal’s intensity is directly related to the brightness of light released from the atoms, which allows for direct measurement of sample ion concentration.
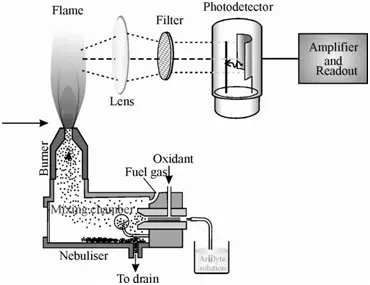
Operating Procedure of a Flame Photometer
The operation of a flame photometer follows a systematic procedure to ensure accurate measurements of metal ion concentrations. Below is a detailed guide on how the flame photometer operates:
- Preparation of Solutions:
Both the standard stock solution and the sample solution should be prepared using fresh distilled water to avoid any contamination or discrepancies. - Flame Calibration:
The flame is calibrated by adjusting the air and gas mixture. After the calibration, allow the flame to stabilize for approximately 5 minutes to ensure consistency. - Instrument Setup:
Switch on the flame photometer and open the filter chamber lids. Insert the appropriate color filters, which are essential for isolating specific wavelengths of light emitted by the atoms. - Zero Calibration:
Before taking measurements, spray distilled water into the flame to set the galvanometer reading to zero. This step ensures that any background signal is accounted for. - Sensitivity Adjustment:
Adjust the sensitivity of the instrument by spraying the most concentrated standard working solution into the flame. Record the full-scale deflection of the galvanometer to set a reference point for the measurements. - Stabilizing Readings:
To achieve stable readings, spray distilled water into the flame again and allow the galvanometer to stabilize. Once stable, readjust the galvanometer to zero. - Standard Solution Measurement:
Spray each standard working solution into the flame three times, recording the galvanometer readings after each spray. Be sure to wash the apparatus thoroughly after each spray to prevent cross-contamination. - Sample Solution Measurement:
Similarly, spray the sample solution into the flame three times, recording the galvanometer readings after each spray. Again, ensure the apparatus is cleaned after each measurement. - Data Analysis:
After obtaining the readings for both the standard solutions and the sample, calculate the mean of the galvanometer readings for each sample. - Graph Plotting:
Plot a graph of concentration versus the galvanometer reading. This graph allows for determining the concentration of the element in the sample based on the recorded readings.
Throughout the procedure, the flame photometer relies on several key processes:
- Desolvation: The solvent in the sample is evaporated by the flame, leaving behind the metal particles.
- Vaporization: The metal particles are further heated, causing the solvent to fully evaporate.
- Atomization: The metal ions are converted into metal atoms by the intense heat of the flame.
- Excitation: The heat energy excites the atoms, causing electrons to move to higher energy levels.
- Emission: As the excited atoms return to their ground state, they emit light at characteristic wavelengths, which is measured by the photo-detector.
The Scheibe-Lomakin equation explains the relationship between the intensity of light emitted and the concentration of an element in a sample. It provides a mathematical framework for understanding this dependency.
- Formula Representation:
The equation is expressed as:
I = k × cⁿ
Where:- I represents the intensity of the emitted light.
- c denotes the concentration of the element in the sample.
- k is the proportionality constant.
- n is an exponent that varies depending on the linearity of the system.
- Linear Range of Calibration Curve:
When the calibration curve is in its linear region, n approximates 1.
Under these conditions, the equation simplifies to:
I = k × c - Direct Proportionality:
In the linear range, the emitted light intensity (I) is directly proportional to the concentration (c).
This means that as the concentration increases, the intensity of the emitted light increases proportionally. - Practical Implication:
This relationship is essential for quantitative analyses, particularly when using instruments like flame photometers or similar devices that measure light emission.
| Element | Emitted wavelength | Flame color |
|---|---|---|
| Potassium (K) | 766 nm | Violet |
| Lithium (Li) | 670 nm | Red |
| Calcium (Ca) | 622 nm | Orange |
| Sodium (Na) | 589 nm | Yellow |
| Barium (Ba) | 554 nm | Lime green |
Applications of Flame Photometer
Flame photometry is a versatile analytical technique widely used across various fields for determining the concentrations of elements. Its applications extend to clinical, environmental, industrial, and research domains.
- Clinical Chemistry:
- Applied to measure critical electrolytes such as sodium, potassium, and calcium in biological fluids like blood serum, plasma, and urine.
- Essential for diagnosing and monitoring conditions related to electrolyte imbalances.
- Environmental Analysis:
- Water Quality Testing: Determines elements like magnesium and calcium that affect water hardness in both industrial and natural water sources.
- Soil Analysis: Analyzes soil samples for sodium, potassium, calcium, and magnesium content to guide agricultural practices and land management strategies.
- Industrial Applications:
- Glass and Ceramics Industry: Measures concentrations of sodium, potassium, boron, and lithium during glass manufacturing processes.
- Cement Industry: Quantifies sodium, potassium, calcium, magnesium, and manganese in raw materials to ensure quality control.
- Pharmaceutical Analysis:
- Utilized for quantifying trace elements in pharmaceutical products to maintain quality and comply with health standards.
- Food and Beverage Industry:
- Determines mineral content in food products, aiding in quality control and accurate nutritional labeling.
- Geological Studies:
- Supports mineral exploration and research by measuring elemental concentrations in geological samples.
- Petroleum Products Analysis:
- Detects metals such as lead and manganese in petroleum products like gasoline and lubricants.
- Ash Analysis:
- Routinely used to estimate alkali and alkaline earth metal oxides in ash samples across various industries.
Advantages of Flame Photometer
Flame photometry is a highly regarded method for quantifying alkali and alkaline earth metals due to its precision and practicality. It offers several distinct advantages across scientific and industrial applications.
- High Sensitivity:
- Capable of detecting trace levels of elements such as sodium, potassium, calcium, and lithium.
- Especially valuable in clinical diagnostics and environmental testing where accurate trace measurements are essential.
- Ease of Use:
- Designed with straightforward operational steps, making it accessible to technicians with minimal training.
- Simplifies workflows in laboratories compared to more intricate analytical equipment.
- Rapid Analysis:
- Enables multiple tests to be completed quickly.
- Efficiently handles large sample volumes without extensive preparation or delays typical of other methods.
- Cost-Effectiveness:
- Operates with minimal consumable requirements and avoids costly instrument setups.
- Provides a budget-friendly option for routine elemental analysis in various fields.
- Wide Measurement Range:
- Supports a broad concentration range, allowing flexibility in analyzing diverse sample types.
- Suitable for both trace detection and higher concentration assessments.
- Low Maintenance Needs:
- Requires less upkeep compared to advanced analytical instruments, reducing operational interruptions.
- Minimizes repair costs and ensures consistent functionality over time.
- Established Reliability:
- Recognized as a robust and proven method with extensive documentation and support resources.
- Widely applied in fields like clinical chemistry, environmental monitoring, and industrial quality control.
- Versatile Sample Compatibility:
- Handles various sample types, from biological fluids to environmental and industrial materials.
- Expands its use across multiple disciplines, enhancing its utility for research and routine testing.
Disadvantages of Flame Photometer
While flame photometry offers several advantages, it also has limitations. These limitations may affect its accuracy and range of applications.
- Element Detection Limit: Flame photometry is used to detect elements that emit light at a wavelength in the range of visible and near moving into ultraviolet light. Element Detection Limit Sodalite only coexists with SO (with a few exceptions at Joey) and Sodium, potassium, calcium and lithium is better than any other method yet developed for detecting the contentf these common or alkali metals.
- Interference issues: Going against the grain on occasion, such as with urine quantification of sodium and potassium, problems like matrix effects and spectral interferences can arise from complex materials.
- Substances that interfere with accurate results: A sample consisting of other substances can affect the accuracy of results. That is why the video signal of emission does not come to a standstill.
- Calibration requirements: To obtain accurate results, it is important to calibrate often and use standard solutions. Calibration Standards must be prepared as per the GLP.
- Destructive analysis: In the analysis process, the sample is consumed which means that when using this technique with small or precious samples is fatal rather than beneficial.
- The method is destructive. Sample preservation is not its strong point.
- Single-element focus: While some flame photometers can analyze multiple elements, they are not nearly as effective as more advanced techniques like ICP spectroscopy at simultaneous multi-element analysis. Single element detection is what it does best, although part-by calculation can of course be applied to multiple element samples.
- Type of sample limitation: Flame photometry is used mainly for liquid samples and cannot be used in solid or gaseous materials without further treatment. However, some mountainous rocks will not adhere at all to flame photometry, requiring special Survey drilling procedures.
- Lack of Molecular Information: The technique provides only the total metal content data. It does not say what form of atom or molecular compound the substance is whose elements are being detected.
- Sensitivity to volatile elements: During analysis volatile elements pose a problem. The heating process might cause them to evaporate out of the sample and this is both dangerous for health and toxic, strange analysis would result in a Read Your Life! less sense weürger (1987).
- https://www.chemlabgenius.com/what-is-flame-photometer/
- https://chrominfo.blogspot.com/2018/11/advantages-and-disadvantages-of-flame.html
- https://www.bwbtech.com/post/advantages-and-disadvantages-when-is-the-flame-photometer-the-best-choice
- https://www.firsthope.co.in/flame-photometry-principle
- https://www.mrclab.com/flame-photometer-principle-of-operation
- https://www.studyandscore.com/studymaterial-detail/flame-photometer-principle-components-working-procedure-applications-advantages-and-disadvantages
- https://whatishplc.com/chemistry/principle-and-procedure-of-flame-photometer/
This is a wonderful document. Keep it up. Produce more.