Feeder pathway is the metabolic process in which different non-glucose carbohydrates are changed into intermediates that can enter glycolysis. It is the process that allows fructose, galactose, mannose and stored polysaccharides like glycogen to be converted into common glycolytic compounds. These intermediates then move into the normal glycolytic steps for further breakdown.
In this pathway different substrates is first modified into molecules such as glucose-1-phosphate or triose phosphates. The Leloir pathway changes galactose into glucose-1-phosphate and fructolysis changes fructose into DHAP or other triose molecules. It is the process that helps in utilizing various dietary carbohydrates and stored reserves for energy production through glycolysis.
Feeder Pathways
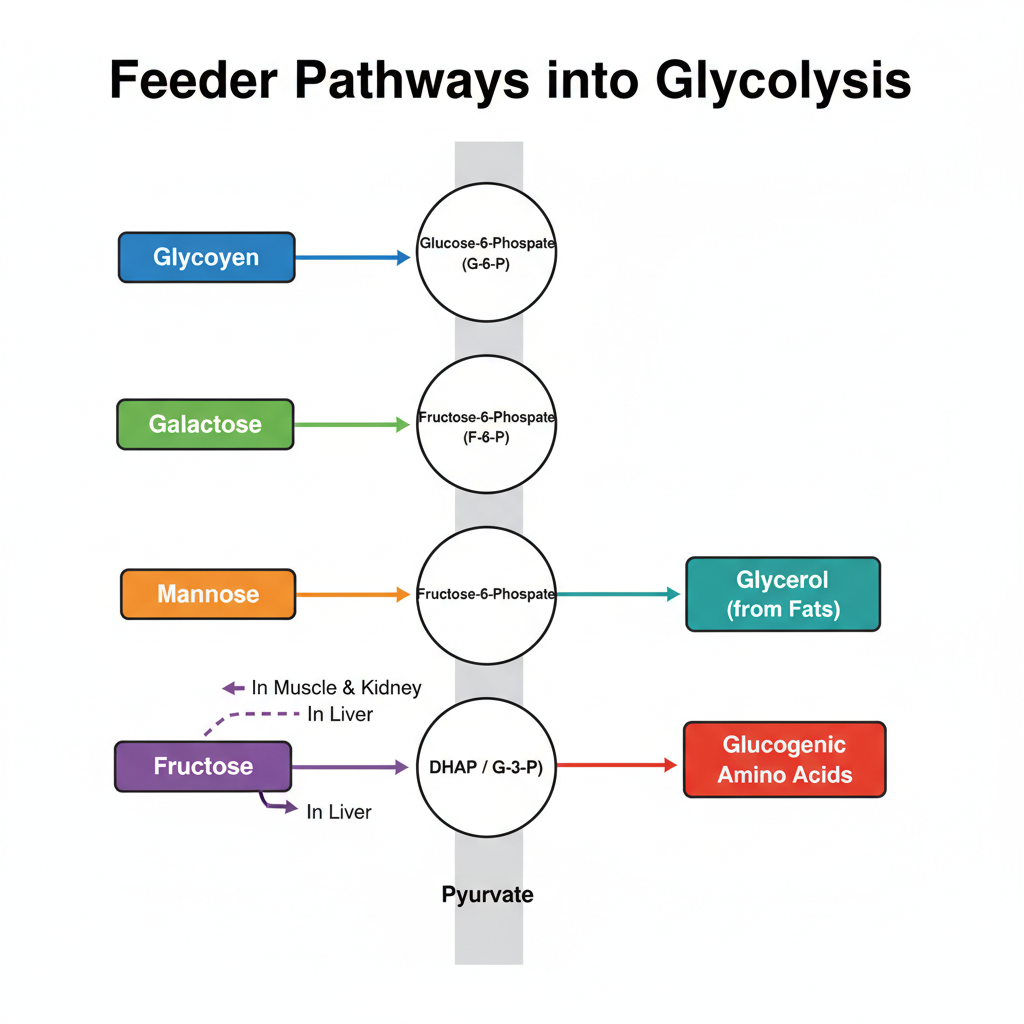
Feeder pathways is the processes by which different carbohydrates and some non-carbohydrate substrates are converted into glycolytic intermediates. It is the process that allow these substrates to enter the main glycolysis pathway. The reactions is enzyme-controlled and each substrate follow a specific sequence of steps.
1. Glycogenolysis (Breakdown of Glycogen)
It is the process of degrading glycogen stored inside the cell. It occurs mainly in liver and muscle tissues. The reaction is as follows– glycogen is cleaved and converted to glucose-1-phosphate.
Steps
- Step 1: Phosphorolytic cleavage– It is catalyzed by glycogen phosphorylase. It is the process where inorganic phosphate (Pi) attack α(1→4) glycosidic bond at the non-reducing end. Glucose-1-phosphate (G-1-P) is formed.
- Step 2: Debranching process– A debranching enzyme act on the branch. It transfer a trisaccharide unit to the linear chain. Then the α(1→6) linkage is hydrolyzed releasing free glucose.
- Step 3: Isomerization– Phosphoglucomutase convert G-1-P into G-6-P. A bisphosphate intermediate is formed during this reaction.
Fate of G-6-P
- In muscle the G-6-P enter glycolysis because muscle do not contain glucose-6-phosphatase.
- In liver the G-6-P is dephosphorylated to glucose and released into blood.
2. Fructose Metabolism (Fructolysis)
Fructose enter glycolysis either through hexokinase or by a special hepatic pathway. It is the process that convert fructose into triose phosphates.
A. In Muscle and Kidney
Fructose is phosphorylated by hexokinase. Fructose-6-phosphate is formed which directly join glycolysis.
B. Hepatic Pathway
- Step 1: Phosphorylation– Fructokinase (KHK) phosphorylate fructose into fructose-1-phosphate (F-1-P). ATP is used.
- Step 2: Cleavage– Aldolase-B splits F-1-P into DHAP and glyceraldehyde.
- Step 3: Phosphorylation of glyceraldehyde– Triokinase convert glyceraldehyde to Glyceraldehyde-3-phosphate (G-3-P).
- Step 4: Entry into glycolysis – DHAP and G-3-P join glycolysis at step 4 and step 5.
3. Galactose Metabolism (Leloir Pathway)
It is the process that convert galactose into glucose derivative. It enters glycolysis as G-6-P.
Steps
- Step 1: Phosphorylation– Galactokinase form galactose-1-phosphate (Gal-1-P).
- Step 2: Uridylyl transfer– GALT exchange the UDP part from UDP-glucose to Gal-1-P. UDP-galactose and G-1-P is obtained.
- Step 3: Epimerization– UDP-galactose is converted to UDP-glucose by epimerase (GALE).
- Step 4: Isomerization– G-1-P is converted to G-6-P by phosphoglucomutase.
4. Mannose Metabolism
It is the process that convert mannose into fructose-6-phosphate.
Steps
- Step 1: Phosphorylation– Hexokinase act on mannose to form mannose-6-phosphate.
- Step 2: Isomerization– Phosphomannose isomerase convert mannose-6-phosphate into fructose-6-phosphate.
5. Non-Carbohydrate Feeders
A. Glycerol Pathway
It is the process where glycerol from fats enter glycolysis.
- Step 1: Phosphorylation– Glycerol kinase convert glycerol into glycerol-3-phosphate.
- Step 2: Oxidation– Glycerol-3-phosphate dehydrogenase convert it into DHAP which is a glycolytic intermediate.
B. Glucogenic Amino Acids
These amino acids is converted into pyruvate or TCA intermediates.
Mechanism– Amino acids are deaminated. α-keto acids enter TCA cycle and converted to oxaloacetate (OAA). OAA is converted to phosphoenolpyruvate (PEP) by PEPCK.
Alanine is transaminated directly to pyruvate.
1. Glycogenolysis: Intracellular Glycogen Breakdown
- It is the process of breaking down intracellular glycogen into glucose-1-phosphate or free glucose.
- It is mainly occurring in liver and skeletal muscle where liver helps in maintaining blood glucose level and muscle provide energy for contraction.
- Glycogenolysis uses phosphorolysis instead of hydrolysis and this helps in conserving energy of the glycosidic bond.
- The breakdown takes place in cytosol and involves few important enzymatic steps for linear chain and branch chain.
- Glycogen phosphorylase act first and it cleaves α(1→4) linkages at non-reducing ends by using inorganic phosphate (Pi).
- The product formed is glucose-1-phosphate (G1P) and it is the main product of this step.
- The enzyme stops when four glucose residues is left near an α(1→6) branch point and this is called limit dextrin.
- The debranching enzyme act now and it has two activities needed to complete the breakdown.
- Transferase activity moves three glucose residues from the branch to a nearby chain exposing the α(1→6) linkage.
- Glucosidase activity hydrolyses the α(1→6) bond releasing free glucose.
- Most of the G1P is changed into glucose-6-phosphate (G6P) by phosphoglucomutase.
- The fate of G6P depends on the tissue in which the process is taking place.
- In liver it is converted to free glucose by glucose-6-phosphatase and released into blood.
- In muscle there is no glucose-6-phosphatase so G6P enter glycolysis directly for ATP production.
- Hormones regulate glycogenolysis and glucagon stimulate the liver while epinephrine stimulate the muscle.
- Insulin inhibit glycogenolysis when blood sugar is high.
- Allosteric regulation also occur where AMP activate glycogen phosphorylase in muscle and ATP inhibit it.
- Ca2+ released during contraction activate phosphorylase kinase linking muscle activity with glycogen breakdown.
- A small part of cellular glycogen is degraded inside lysosomes by acid α-glucosidase.
- Defects in these enzymes cause glycogen storage diseases like Von Gierke disease, Pompe disease, Cori disease and McArdle disease.
2. Fructose Metabolism (Fructolysis)
- It is the process where fructose is broken down inside the cell and converted into intermediate compounds that enter glycolysis and other metabolic pathways.
- It is mainly occurring in the liver because the liver has the enzymes needed for fructolysis.
- Fructose enters the cell through GLUT5 transporter by facilitated diffusion.
- The liver take most of the fructose load while small amount is metabolised in intestine and kidney.
- In muscle the metabolism is very less because GLUT5 is low and hexokinase does not phosphorylate fructose effectively.
- The first step is phosphorylation where fructokinase (Ketohexokinase) convert fructose into fructose-1-phosphate (F1P).
- This step use ATP and fructokinase is not regulated by feedback so the reaction is rapid.
- Due to rapid phosphorylation ATP and inorganic phosphate is depleted inside the cell.
- Aldolase B act next and it cleaves F1P into dihydroxyacetone phosphate (DHAP) and glyceraldehyde.
- DHAP is a glycolytic intermediate and can enter the main pathway easily.
- Glyceraldehyde is phosphorylated by triokinase to form glyceraldehyde-3-phosphate (G3P).
- Both DHAP and G3P now enter glycolysis after step 4 bypassing the phosphofructokinase (PFK-1) control point.
- This bypass is important because the cell cannot regulate fructose breakdown at this step.
- Excess triose phosphates convert to pyruvate and then acetyl CoA leading to more fatty acid formation.
- This is the reason high fructose intake is linked to hepatic fat accumulation and high triglycerides.
- Fructose carbon can be used for glycogen synthesis when glycogen is low.
- A large part of fructose may convert into lactate which is released into blood.
- This lactate can be used by muscle for energy production.
- Rapid ATP loss during fructolysis activate AMP deaminase and AMP is converted into uric acid.
- Rise in uric acid level is a common effect of high fructose intake.
- Small intestine also metabolise fructose and convert it to glucose or lactate before reaching liver.
- When intestinal capacity is exceeded the extra fructose goes to liver.
- Muscle can convert fructose to fructose-6-phosphate by hexokinase but this occurs only when glucose is absent or very low.
- Genetic defects in this pathway cause specific disorders.
- Essential fructosuria occur due to lack of fructokinase and is generally harmless.
- Hereditary fructose intolerance is caused by aldolase B deficiency.
- F1P accumulate in the liver and inhibit glycogenolysis and gluconeogenesis.
- Severe hypoglycemia, vomiting and liver damage is seen in this condition.
- Fructose accumulation in intestine may cause ER stress affecting protein processing.
- This can reduce tight junction proteins and lead to leaky gut and more inflammation.
3. Galactose Metabolism (The Leloir Pathway)
- It is the process where galactose obtained from lactose breakdown is converted into glucose-1-phosphate.
- Galactose cannot enter glycolysis directly so it must be changed into a glycolytic intermediate through few enzymatic steps.
- Lactose is hydrolysed into glucose and β-D-galactose first.
- Galactose mutarotase convert β-D-galactose into α-D-galactose because galactokinase act only on α-form.
- Galactokinase phosphorylate galactose at C-1 position forming galactose-1-phosphate (Gal-1P).
- This step use ATP and it is the committed step of the pathway.
- Galactose-1-phosphate uridylyltransferase (GALT) act next and transfer UMP from UDP-glucose to Gal-1P.
- This reaction form UDP-galactose and release glucose-1-phosphate.
- UDP-galactose is then converted back to UDP-glucose by UDP-galactose-4-epimerase (GALE).
- This epimerization is needed to maintain the supply of UDP-glucose for continuous cycling.
- Glucose-1-phosphate produced is changed into glucose-6-phosphate by phosphoglucomutase.
- The glucose-6-phosphate now enter glycolysis or it may be used for glycogen synthesis.
- The pathway also produce UDP-sugars which are important for forming glycoproteins and glycolipids.
- Enzyme defects in this pathway cause galactosemia in newborns.
- Classic galactosemia occur due to GALT deficiency and Gal-1P accumulate in tissues.
- This cause liver enlargement, poor growth and can be fatal if galactose is not removed from diet.
- Galactokinase deficiency cause increase galactose level and cataract formation due to galactitol.
- Epimerase deficiency show variable symptoms and may resemble GALT deficiency in severe form.
- Management of these disorders require strict removal of galactose from food.
4. Mannose Metabolism
- It is the process where mannose, a hexose monosaccharide, is used as a feeder substrate for glycolysis.
- Mannose is obtained from digestion of different glycoproteins and polysaccharides present in food.
- In microorganisms like yeast it is fermented along with glucose and fructose through the EMP pathway.
- The metabolism occur in two main enzymatic steps inside the cell.
- Hexokinase act first and it phosphorylate mannose into mannose-6-phosphate using ATP.
- This step is similar to glucose phosphorylation but the product formed is mannose-6-phosphate.
- Phosphomannose isomerase act next and it convert mannose-6-phosphate into fructose-6-phosphate.
- Fructose-6-phosphate is a normal glycolytic intermediate so mannose enter glycolysis at this point.
- It enter glycolysis after the conversion of glucose to glucose-6-phosphate.
- This position is upstream of the regulatory enzyme PFK-1 so mannose metabolism follow the normal glycolytic control.
- Once mannose-derived carbon becomes fructose-6-phosphate it is used for ATP production or other metabolic needs.
- This feeder pathway help in utilizing carbohydrate portions of dietary glycoproteins and polysaccharides efficiently.
5. Non-Carbohydrate Feeders
Non-carbohydrate feeder pathways allow the body to generate energy or synthesize glucose from non-sugar substrates, primarily lipids (via glycerol) and proteins (via amino acids), as well as metabolic byproducts like lactate. These pathways are essential during periods of fasting, starvation, or intense exercise when carbohydrate stores are depleted.
- Glycerol Metabolism (Lipid–Derived)
- It is the three-carbon glycerol molecule released during lipolysis of triglycerides in adipose tissue.
- Glycerol is glucogenic and it is mainly utilized in liver and kidney because these tissues have high glycerol kinase activity.
- In this step glycerol is phosphorylated by glycerol kinase forming glycerol-3-phosphate.
- Glycerol-3-phosphate is then oxidized by glycerol-3-phosphate dehydrogenase to form DHAP (Dihydroxyacetone phosphate).
- DHAP is an intermediate of glycolysis and this enters the pathway after PFK-1 so the major regulatory step is bypassed.
- During fasting the DHAP is mostly used in gluconeogenesis to maintain the blood glucose level.
- Glucogenic Amino Acids (Protein–Derived)
- These amino acids is converted into glucose precursors during prolonged fasting when carbohydrate reserves is low.
- The carbon skeleton is first deaminated forming α-ketoacids and these enters the TCA cycle at different points like α-ketoglutarate or succinyl-CoA.
- These intermediates is converted into oxaloacetate (OAA) and OAA is changed into PEP by PEPCK which allows entry into gluconeogenesis.
- The alanine cycle is another important process. In this cycle alanine from skeletal muscle is transported to liver and is transaminated to pyruvate.
- This pyruvate is used as substrate for gluconeogenesis and it helps in transferring carbon from muscle to liver.
- Lactate (Cori Cycle)
- Lactate is produced in anaerobic glycolysis especially in skeletal muscle during vigorous exercise and in RBCs due to absence of mitochondria.
- It is transported through the blood to the liver. In liver lactate is converted back to pyruvate by lactate dehydrogenase.
- This pyruvate is utilized in gluconeogenesis to form glucose.
- The glucose is released to circulation again and is used by peripheral tissues. This repeated cycling of lactate and glucose is referred to as the Cori cycle.
Importance of Feeder Pathways
- It is important as these pathways expand the metabolic fuel pool by allowing different monosaccharides and some non-carbohydrate molecules to enter glycolysis.
- It is the process that helps the cell to utilize fructose, galactose, mannose and even glycerol or some amino acids by converting them into glycolytic intermediates.
- Dietary substrates like fructose and galactose is changed into glucose-6-phosphate or fructose-6-phosphate, so these are used for ATP generation.
- Glycerol from fat breakdown is converted to DHAP and enter the pathway, which is helpful during fasting when glucose is less.
- Glycogenolysis is important because glucose-1-phosphate is formed without ATP use, giving more net ATP in glycolysis.
- In muscle, this process provide rapid energy as glycogen is immediately broken and fuel is supplied during sudden activity.
- Entry of substrates upstream or downstream of PFK-1 controls how these molecules is regulated.
- When the substrate enters before PFK-1 (like galactose or glycogen), the breakdown is regulated by ATP and citrate levels.
- When fructose enters after PFK-1, it bypass this regulation so the metabolism is very fast and lead to accumulation of acetyl-CoA.
- This unregulated flow is the major source of increased fat synthesis inside liver.
- Feeder pathways also show tissue-specific function.
- In liver, G-6-phosphatase release free glucose into blood to maintain glucose level for other tissues.
- Muscle glycogen is used only inside muscle because the enzyme for releasing free glucose is absent.
- Defects in these pathways is associated with accumulation of toxic intermediates like fructose-1-phosphate or galactose-1-phosphate.
- These accumulated products interfere with ATP formation and cause liver damage or hypoglycemia.
- Excessive fructose metabolism is associated with lipid accumulation, inflammation and metabolic disorders.
- Chandel, N. S. (2021). Glycolysis. Cold Spring Harbor Perspectives in Biology, 13(5), a040535. https://doi.org/10.1101/cshperspect.a040535
- Chaudhry, R., & Varacallo, M. A. (2023, August 8). Biochemistry, Glycolysis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482303/
- Ganapathy, V. (n.d.). GLYCOLYSIS [Lecture notes]. Texas Tech University Health Sciences Center. https://www.ttuhsc.edu/medicine/academic-affairs/documents/sakai-files/bct/7_Glycolysis_Notes_Ganapathy.pdf
- Garden and Plate. (n.d.). Fructolysis. https://gardenandplate.com/fructolysis.html
- Hannou, S. A., Haslam, D. E., McKeown, N. M., & Herman, M. A. (2018). Fructose metabolism and metabolic disease. The Journal of Clinical Investigation, 128(2), 545–555. https://doi.org/10.1172/JCI96702
- Hartley, C. J., French, N. G., Scoble, J. A., Williams, C. C., & Scott, C. (2017). Sugar analog synthesis by in vitro biocatalytic cascade: A comparison of alternative enzyme complements for dihydroxyacetone phosphate production as a precursor to rare chiral sugar synthesis. PLOS ONE, 12(11), e0187994. https://doi.org/10.1371/journal.pone.0187994
- Integration of Feeder Pathways into Glycolysis: A Comprehensive Analysis of Substrate Flux and Metabolic Regulation. (n.d.). [Provided source text].
- Melkonian, E. A., Asuka, E., & Schury, M. P. (2023, November 13). Physiology, Gluconeogenesis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK541119/
- Pan, S. (2024, March 29). Feeder Pathways for Glycolysis. Biology Notes Online.
- Patino, S. C., & Orrick, J. A. (2024, January 27). Biochemistry, Glycogenolysis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK549820/
- Ross, K. L., Davis, C. N., & Fridovich-Keil, J. L. (2004). Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Molecular Genetics and Metabolism, 83(1-2), 103–116. https://doi.org/10.1016/j.ymgme.2004.07.005
- Tappy, L. (2017). Fructose Metabolism from a Functional Perspective: Implications for Athletes. Sports Science Exchange, 28(174), 1–5. https://www.gssiweb.org/sports-science-exchange/article/fructose-metabolism-from-a-functional-perspective-implications-for-athletes
- Taylor & Francis. (n.d.). Leloir pathway. Taylor & Francis Knowledge. https://taylorandfrancis.com/knowledge/Medicine_and_healthcare/Physiology/Leloir_pathway/
- Todoric, J., Di Caro, G., Reibe, S., Henstridge, D. C., Green, C. R., Vrbanac, A., Ceteci, F., Conche, C., McNulty, R., Shalapour, S., Taniguchi, K., Meikle, P. J., Watrous, J. D., Moranchel, R., Najhawan, M., Jain, M., Liu, X., Kisseleva, T., Diaz-Meco, M. T., … Karin, M. (2020). Fructose stimulated de novo lipogenesis is promoted by inflammation. Nature Metabolism, 2(10), 1034–1045. https://doi.org/10.1038/s42255-020-0261-2
- Webb, B. A., Dosey, A. M., Wittmann, T., Kollman, J. M., & Barber, D. L. (2017). The glycolytic enzyme phosphofructokinase-1 assembles into filaments. The Journal of Cell Biology, 216(8), 2305–2313. https://doi.org/10.1083/jcb.201701084
- Wikipedia contributors. (n.d.). Fructolysis. Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Fructolysis