The fate of pyruvate is the different metabolic pathway in which pyruvate can enter after it is formed at the end of glycolysis. It is the process that depend on the presence or absence of oxygen in the cell environment. In aerobic condition, pyruvate is moved into the mitochondria where it is converted into Acetyl-CoA, CO₂ and NADH.
This step is irreversible and it is catalyzed by pyruvate dehydrogenase complex. It is the link reaction between glycolysis and the citric acid cycle, and the Acetyl-CoA produced is further oxidised for release of large amount of energy through oxidative phosphorylation.
In the absence of oxygen the pyruvate remain in the cytoplasm and it is changed into lactate by the enzyme lactate dehydrogenase. This is referred to as anaerobic glycolysis and it helps in regenerating NAD⁺ for continuation of glycolysis.
In some organism like yeast, this process occur in the form of alcoholic fermentation where pyruvate is converted into ethanol and CO₂. Pyruvate is also important precursor molecule because it is changed into oxaloacetate (OAA) by pyruvate carboxylase in a reaction that require ATP. This process occur when gluconeogenesis is taking place and also helps in maintaining the intermediates of the TCA cycle.
Pyruvate can also undergo transamination reaction to form alanine depending on the requirement of amino acid synthesis.
What are the Products of Glycolysis?
During glycolysis, a single glucose molecule is enzymatically broken down inside the cytosol. It is the process where glucose (6-carbon) is converted into two 3-carbon compounds. The overall reaction is represented as:
Glucose → 2 Pyruvate (or 2 Lactate) + 2 ATP + 2 NADH + 2 H₂O + 2 H⁺
This pathway is also referred to as the Embden–Meyerhof–Parnas pathway and it is carried out in both aerobic and anaerobic conditions. The major products formed at the end of the pathway are described below.
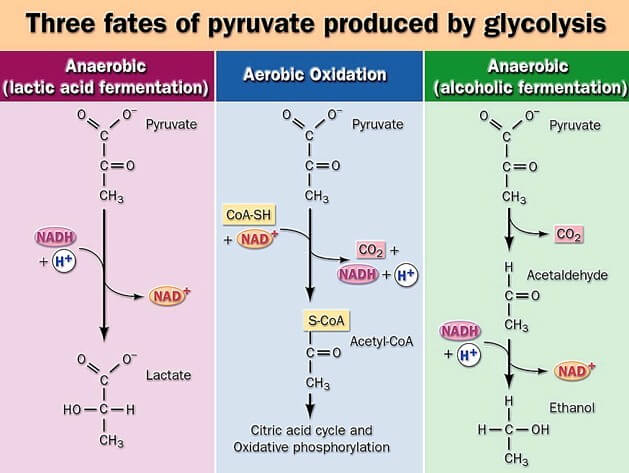
1. Pyruvate (or Lactate)
Two molecules of pyruvate is formed as the terminal product in aerobic glycolysis. It is a 3-carbon α-keto acid and it is positioned at an important metabolic junction. Pyruvate enters into further oxidative processes such as the Krebs cycle when oxygen is available.
In anaerobic condition, pyruvate is reduced to lactate and thus 2 molecules of lactate is the product.
2. ATP (Adenosine Triphosphate)
A total of four ATP molecules is generated in the payoff phase. But two ATP is used in the initial steps, therefore the net gain is 2 ATP only. It is the energy unit required for supporting different metabolic activities inside the cell. These ATP is formed by substrate-level phosphorylation.
3. NADH (Nicotinamide Adenine Dinucleotide)
Two molecules of NADH is formed when glyceraldehyde-3-phosphate is oxidised. This NADH acts as a reduced electron carrier and it is further used for ATP generation inside mitochondria under aerobic condition. In anaerobic type it is reoxidised back during formation of lactate.
4. Water (H₂O)
Two molecules of water is released during the conversion of 2-phosphoglycerate into phosphoenolpyruvate. This step removes water from the substrate.
5. Protons (H⁺)
Two H⁺ ions is formed mainly along with NADH production. These protons participate in maintaining redox balance.
What is the fate of the pyruvate?
Pyruvate formed at the end of glycolysis is a 3-carbon compound and it is the point where different metabolic routes is decided inside the cell. It is the process in which pyruvate enters either aerobic or anaerobic type depending on oxygen availability.
In aerobic condition, pyruvate is transported into mitochondria where it is converted to acetyl CoA by oxidative decarboxylation. This acetyl CoA now enters the Krebs cycle for further oxidation and energy release. In another aerobic reaction, pyruvate may also be carboxylated to form oxaloacetate, and this is an important intermediate for many biosynthetic processes.
Under anaerobic condition, the fate is different because the pyruvate is reduced to lactate, and this reaction helps in regenerating NAD⁺ for continuation of glycolysis. In some organisms, pyruvate is changed into ethanol and carbon dioxide when oxygen is absent. Thus, it is seen that pyruvate acts as a central metabolite and its conversion into acetyl CoA, oxaloacetate, lactate, or ethanol depends on the pathway in which the cell is operating.
A. Fate of Pyruvate in Presence of oxygen
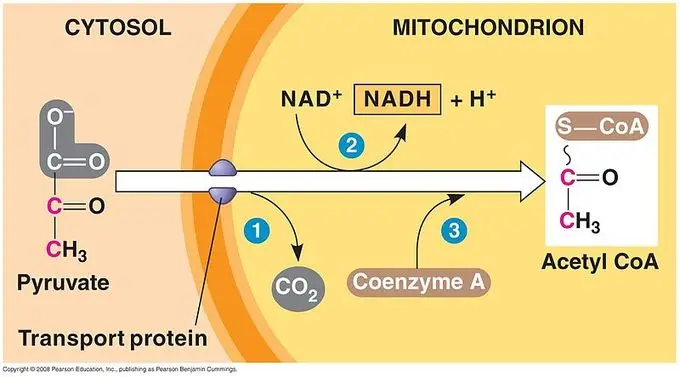
In aerobic condition, pyruvate formed at the end of glycolysis is not allowed to enter directly into the TCA cycle. It is first converted into some intermediate compounds which can take part in the cycle. These reactions occur inside mitochondria and it is the important link between glycolysis and aerobic respiration.
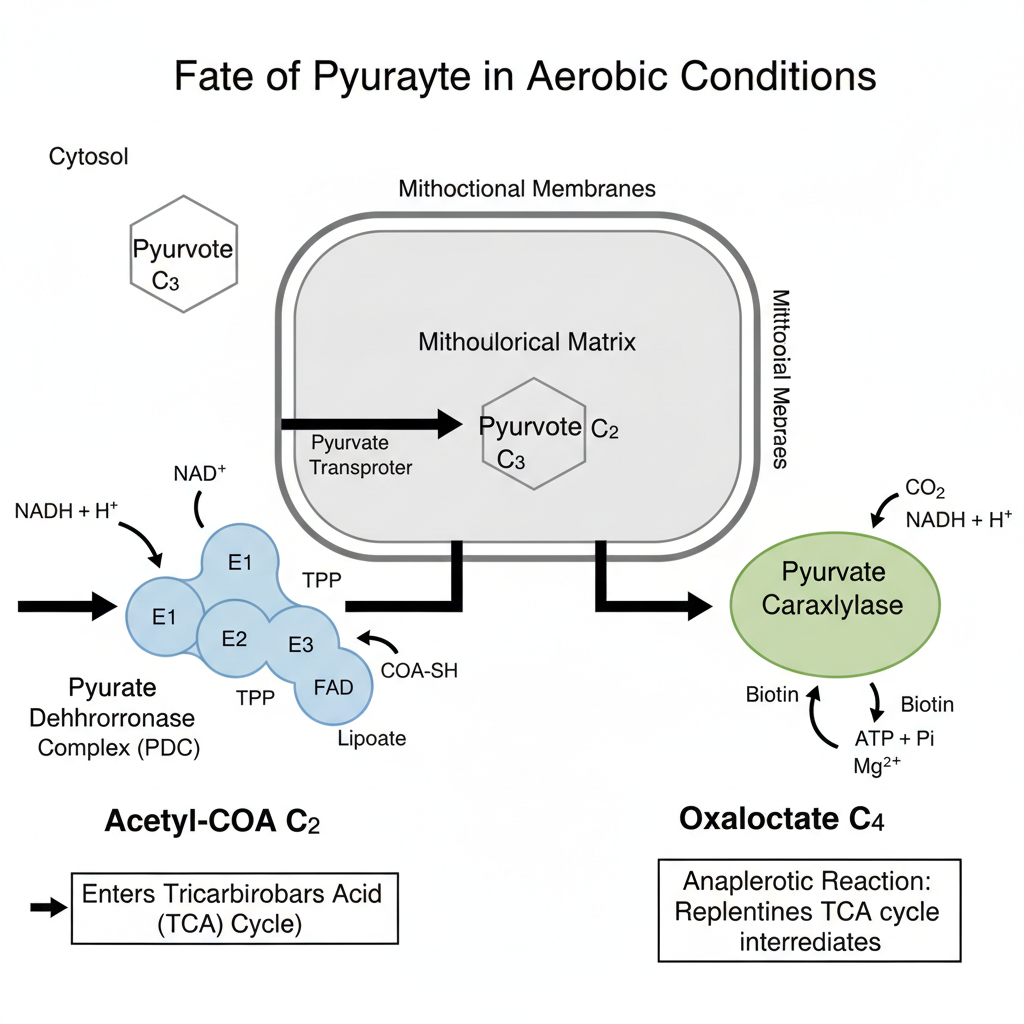
1. Acetyl CoA Formation
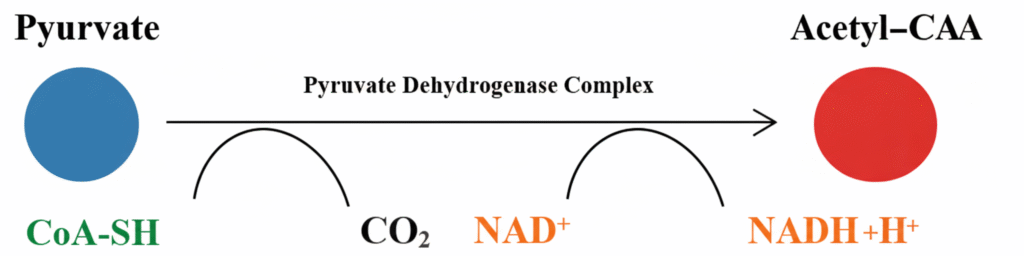
It is the main fate of pyruvate under the presence of oxygen. Pyruvate is transported into the mitochondrial matrix where it is converted into Acetyl CoA. This conversion is catalysed by the “pyruvate dehydrogenase complex (E1, E2, E3)”. It is dependent on 5 coenzymes which are TPP (Thiamine pyrophosphate), FAD (Flavin adenine dinucleotide), lipoate, CoA-SH and NAD⁺.
In this step, decarboxylation takes place by the action of E1 (pyruvate dehydrogenase) with TPP, where one carbon of pyruvate is released in the form of CO₂. The remaining acetyl group is transferred to CoA-SH by E2 (dihydrolipoyl transacetylase) and lipoate complex, forming Acetyl CoA. The regeneration of oxidised lipoate is carried out by E3 (dihydrolipoyl dehydrogenase) which transfers hydrogen to FAD and then to NAD⁺ forming NADH + H⁺.
Acetyl CoA is the final product of this oxidative decarboxylation and it enters the TCA cycle for further oxidation.
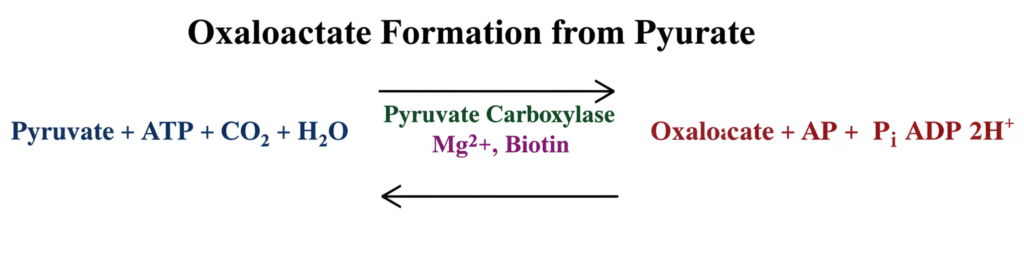
2. Oxaloacetate Formation
Pyruvate may also be converted into oxaloacetate when oxygen is present. This reaction is catalysed by pyruvate carboxylase enzyme. It is a carboxylation process and requires biotin along with Mg²⁺ ions. A molecule of ATP is utilized in this reaction.
Oxaloacetate formation is an important anaplerotic process because it replenishes the intermediates of the TCA cycle. It is also the beginning step of gluconeogenesis. The reaction occurs in the mitochondrial matrix and it helps in maintaining the continuous operation of the TCA cycle.
Thus, in aerobic condition pyruvate is not wasted. It is converted either to Acetyl CoA for energy production or to oxaloacetate to maintain metabolic balance inside mitochondria.
B. Fate of Pyruvate in Absence of oxygen
In absence of oxygen the pyruvate formed in glycolysis is not oxidised further in mitochondria. It is the condition where the cell depends on simple reduction reactions to maintain the supply of NAD⁺ so that glycolysis can continue. These reactions occur in cytoplasm and the products formed are different in different organisms.
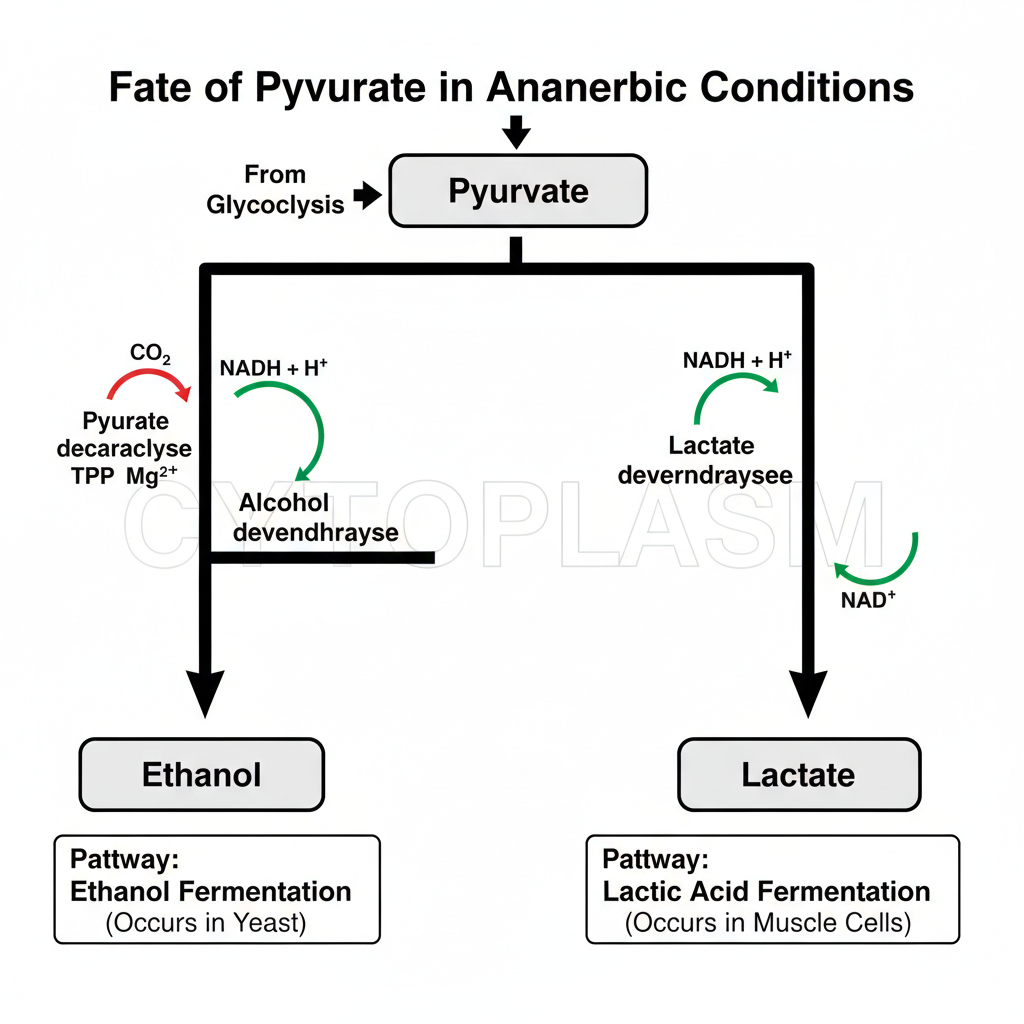
1. Ethanol Formation
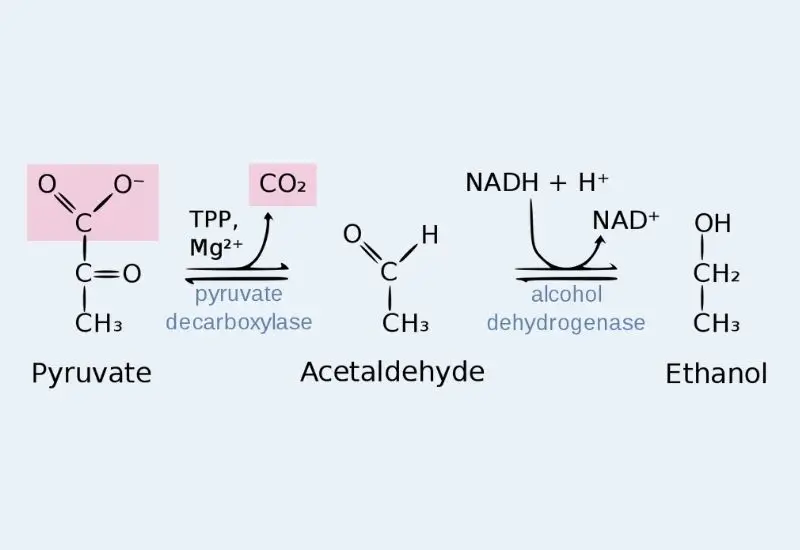
It is the process seen in yeasts and some microorganisms. Pyruvate is converted to ethanol in two simple steps. In the first step pyruvate is decarboxylated to form acetaldehyde. The enzyme is pyruvate decarboxylase and it requires TPP (Thiamine pyrophosphate) and Mg²⁺ ion. In this step CO₂ is released from pyruvate. It is the main decarboxylation process.
In the next step the acetaldehyde is reduced into ethanol. This reaction is carried out by alcohol dehydrogenase. NADH is oxidised to NAD⁺ which helps in continuing the glycolysis. It is the basic fermentation reaction occurring in anaerobic condition.

2. Lactate Formation
In many tissues, when oxygen supply is low, pyruvate is reduced to lactate. The reaction is catalysed by lactate dehydrogenase. NADH is oxidised to NAD⁺ and this oxidation is important because it maintains the NAD⁺ level for glycolysis. This process occurs in skeletal muscles during repeated contraction, in brain cells, retina, RBCs and in microbial cells also.
The reduction of pyruvate to lactate is simple anaerobic pathway and it is used when the cells cannot use the oxidative pathway.

Biosynthetic Pathway
- Pyruvate can further infiltrate into the biosynthetic pathways for example fatty acids biosynthesis and gluconeogenesis.
- Pyruvate converted into acetyl CoA by the activity of pyruvate dehydrogenase complex this acetyl CoA further enters into the biosynthetic pathway alongside TCA.
- Pyruvate can further infiltrate into gluconeogenesis by the activity of pyruvate carboxykinase transforming it into oxaloacetate which with several steps reaction transforms into glucose. When the glucose level of cells decreased they activate the gluconeogenesis pathway to generate glucose from Pyruvate.
Factors Influencing Pyruvate’s Fate
The fate of pyruvate is controlled by different internal conditions of the cell. It is the point where the pathway may move towards complete oxidation or it may move towards reduction or into some synthetic reactions. Some of the important factors are as follows–
- Availability of oxygen (O₂)– It is the major deciding factor. In presence of oxygen the pyruvate is taken into mitochondria and it is converted to Acetyl CoA by pyruvate dehydrogenase complex for entering the TCA cycle. In absence of oxygen pyruvate stays in cytoplasm and it is reduced into lactate or ethanol for regenerating NAD⁺ needed in glycolysis.
- Presence of mitochondria– Cells which have mitochondria can oxidise pyruvate. Tissues like RBCs which lack mitochondria cannot send pyruvate into TCA cycle. These cells convert pyruvate mainly into lactate. The entry of pyruvate into mitochondria depends on mitochondrial pyruvate carrier (MPC).
- Energy level and redox state of the cell– When ATP, NADH and Acetyl CoA level is high the pyruvate dehydrogenase complex is inhibited and oxidation of pyruvate decreases. When ATP is low and NAD⁺ level increases the complex becomes active and pyruvate is oxidised. High Acetyl CoA activates pyruvate carboxylase and pyruvate is changed into oxaloacetate.
- Hormonal and nutritional condition– Pyruvate dehydrogenase is regulated by phosphorylation. Pyruvate dehydrogenase kinase inactivates the enzyme whereas pyruvate dehydrogenase phosphatase activates it. Insulin activates the enzyme. In starvation the kinase level increases and oxidation of pyruvate is reduced.
- Tissue requirement– In liver and kidney pyruvate is converted into oxaloacetate for gluconeogenesis. In adipose tissue it is used in fatty acid formation. In muscle during continuous contraction pyruvate is reduced to lactate. In many cancer cells more pyruvate is converted to lactate.
- Presence of inhibitors and activators– Some compounds inhibit the mitochondrial pyruvate carrier and reduce aerobic use of pyruvate. Some inhibitors of pyruvate dehydrogenase kinase activate the complex and increase oxidation. In microorganisms some reactive compounds may inhibit pyruvate decarboxylase.
These factors control the direction of metabolism of pyruvate inside the cell.
- Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014 Jul;71(14):2577-604. doi: 10.1007/s00018-013-1539-2. Epub 2013 Dec 21. PMID: 24363178; PMCID: PMC4059968.
- Aaron D. Freedman, Leonard Kohn, Pyruvate Metabolism and Control: Factors Affecting Pyruvic Carboxylase Activity.Science145,58-60(1964).DOI:10.1126/science.145.3627.58
- KOEPPE RE, MOURKIDES GA, HILL RJ. Some factors affecting routes of pyruvate metabolism in rats. J Biol Chem. 1959 Sep;234:2219-22. PMID: 14410462.
- https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_(Boundless)/05:_Microbial_Metabolism/5.03:_Catabolism/5.3B:_Pyruvic_Acid_and_Metabolism
- https://reactome.org/content/detail/R-HSA-70268
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4059968/
- https://pubchem.ncbi.nlm.nih.gov/pathway/Reactome:R-HSA-70268
- https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/pyruvic-acid
- https://www.journalofdairyscience.org/article/S0022-0302(64)88927-X/pdf
- https://www.mdpi.com/2218-273X/10/7/1068
- https://www.sciencedirect.com/science/article/pii/S002192581869787X/pdf?md5=3f5acb008c02f535197d4b241aa4f4a5&pid=1-s2.0-S002192581869787X-main.pdf
- https://www.science.org/doi/10.1126/science.145.3627.58
- https://en.wikipedia.org/wiki/Pyruvate