What is ELISA?
ELISA, short for enzyme-linked immunosorbent assay, is a widely used and highly sensitive test in the field of immunoassays. It was first introduced by Eva Engvall and Peter Perlmann in 1971 and has since become a gold standard technique for detecting and quantifying various molecules, including hormones, glycoproteins, proteins, antibodies, and antigens.
The basic principle behind ELISA involves the interaction between an antibody and an antigen to identify specific molecules. The test is typically performed on a plate, where the antigens present in the sample are immobilized and bound to the surface. This is achieved by attaching the antigens to a solid surface, often a polystyrene plate.
Once the antigens are immobilized, a matching antibody linked to an enzyme is added to the plate. The antibody binds specifically to the antigen, forming an antigen-antibody complex. After allowing sufficient time for the complex to form, the plate is washed to remove any unbound antibodies.
In the final step, a substance known as a substrate for the enzyme is added to the plate. When the substrate interacts with the enzyme, it produces a measurable signal, often a color change or the generation of light. The intensity of the signal is directly proportional to the amount of antigen present in the sample, allowing for quantification.
ELISA can be performed in different ways depending on the specific molecule being detected and the nature of the antibody-antigen interaction. There are four major types of ELISA:
- Direct ELISA: In this method, the antigen is directly immobilized onto the solid surface of the plate. The antibody, conjugated with an enzyme, binds directly to the antigen.
- Sandwich ELISA: This method is used when the antigen of interest is present in low concentrations. It involves coating the plate with a capture antibody that specifically binds to the antigen. The sample containing the antigen is then added, allowing the antigen to bind to the capture antibody. A detection antibody, also conjugated with an enzyme, is subsequently added to bind to the antigen, forming a “sandwich” structure.
- Reverse ELISA: In this variation, the antigen is attached to the solid surface, while the enzyme-linked antibody is present in the sample. The antibody binds to the antigen, and after washing off any unbound components, a substrate is added to generate a signal.
- Competitive ELISA: This method is used when the antigen in the sample competes with a known labeled antigen for binding to a limited amount of antibodies. The signal generated is inversely proportional to the concentration of the antigen in the sample.
ELISA is a powerful and versatile technique, but it requires skilled technicians and specialized equipment. It is known for its high sensitivity and specificity, making it a valuable tool in research, diagnostics, and screening for various diseases, including the detection of HIV antibodies, for which it was initially developed. However, ELISA can be time-consuming due to the incubation and washing steps involved in the process.
When to use ELISA
ELISA is a useful technique for protein analysis in specific situations. Here are some instances when ELISA is commonly employed:
- Detection of Small Amounts or Low-Abundance Proteins: ELISA is highly sensitive and can detect very small amounts of target proteins, including rare or low-abundance proteins. It allows researchers to accurately identify and quantify proteins even when they are present in low concentrations.
- Measurement of Protein Concentration: If you need to determine the exact amount of a specific protein in a sample, ELISA is a valuable tool. It enables the quantitative measurement of protein concentration, which is particularly useful for research involving protein quantification or monitoring changes in protein levels over time.
- Time Constraints in the Laboratory: ELISAs are known for their rapidity and ease of use. They can be performed relatively quickly with minimal sample preparation, making them a convenient choice for routine assays or when time is a limiting factor in the laboratory. ELISA’s efficiency and straightforward procedure make it an efficient option when timely results are required.
By considering these factors, researchers can determine whether ELISA is the appropriate technique for their protein analysis needs. Its high sensitivity, ability to measure protein concentration, and efficiency in time-critical situations make it a valuable tool in various research applications.
What is Western Blot?
Western blot, also known as western blotting, is a widely used analytical technique for the separation and detection of specific proteins from a mixture. It involves a series of steps, including gel electrophoresis, transfer of proteins to a solid membrane, and visualization of the proteins using antibodies.
The technique was first described by Harry Towbin in 1979 and later named by W. Neal Burnette in 1981. The procedure of western blotting can be summarized as follows:
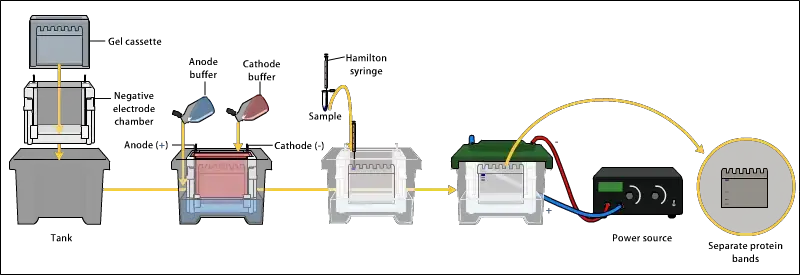
- Gel Electrophoresis: Proteins are separated based on their size using gel electrophoresis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is commonly used for this step. The proteins are loaded onto a gel matrix and subjected to an electric field, causing them to migrate based on their molecular weight.
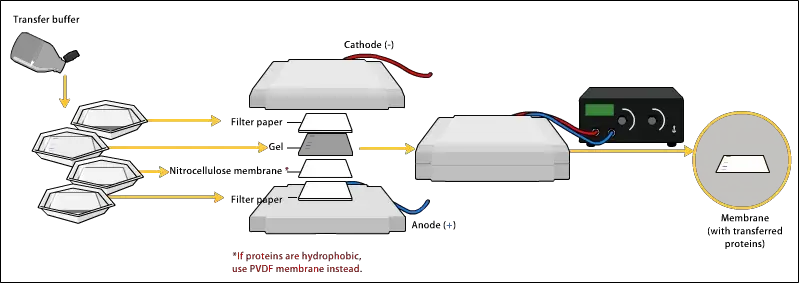
- Transfer: After separation on the gel, the proteins are transferred from the gel to a solid support membrane. Nitrocellulose or polyvinylidene difluoride (PVDF) membranes are commonly used. This transfer, known as electroblotting or western blotting, allows the proteins to retain their relative positions on the membrane.
- Total Protein Staining: The transferred proteins on the membrane are visualized using staining dyes such as Ponceau S, Coomassie Brilliant Blue, or amido black. This step provides a general overview of the protein bands on the membrane and helps confirm successful protein transfer.
- Blocking: To prevent non-specific binding of antibodies, the membrane is incubated with a blocking agent, typically a protein-based solution like milk or bovine serum albumin (BSA). This step blocks any remaining unoccupied binding sites on the membrane.
- Incubation: Primary antibodies specific to the protein of interest are added to the membrane and allowed to bind to their target proteins. The primary antibodies recognize and bind to specific epitopes on the target proteins.
- Detection and Visualization: After washing away unbound primary antibodies, secondary antibodies linked to a reporter enzyme, such as horseradish peroxidase (HRP) or alkaline phosphatase (AP), are added. The secondary antibodies bind to the primary antibodies, amplifying the signal. A substrate specific to the reporter enzyme is applied, resulting in a colorimetric or chemiluminescent reaction at the protein bands on the membrane. This reaction allows the visualization and detection of the target proteins.
By analyzing the protein bands on the membrane, researchers can determine the presence, quantity, and size of specific proteins in a sample. Western blotting is commonly used in various fields of research, including molecular biology, biochemistry, immunology, and diagnostics. It is particularly useful for confirming the presence of specific proteins, studying protein expression levels, and detecting antibodies against certain pathogens or diseases, such as HIV, Hepatitis B, or prion diseases like mad cow disease.
When to use Western blotting
Western blotting is a valuable technique that can be used in specific situations for protein analysis. Here are some instances when Western blotting is commonly employed:
- Detection of a Wide Range of Proteins: Western blots are ideal for analyzing complex mixtures of proteins, such as cell lysates. They allow researchers to detect a wide range of proteins simultaneously. To enhance the analysis, fluorescent multiplexing techniques can be used to detect multiple target proteins at once. This enables a more comprehensive understanding of protein expression patterns. Advanced imaging systems like the Azure 400 imager can even detect and image three target proteins using three-color fluorescence simultaneously.
- Identification of Specific Proteins: Western blotting is particularly useful for identifying specific proteins in a sample. It can detect target proteins even when they are present at low levels or within complex mixtures. By using specific antibodies against the protein of interest, Western blotting allows researchers to confirm the presence of the target protein and distinguish it from other proteins present in the sample.
- Confirmation of Protein Identity and Characteristics: Western blots can provide confirmation of the correct protein being measured. By assessing factors such as protein size, Western blots can confirm the presence of the desired protein. Additionally, Western blotting can reveal important information about protein modifications, impurities, or other characteristics that may impact its function or behavior.
In summary, Western blotting is a powerful tool for protein analysis in various research scenarios. It enables the detection of a wide range of proteins, identification of specific proteins within complex mixtures, and confirmation of protein identity and characteristics. By leveraging these capabilities, researchers can gain valuable insights into protein expression, function, and modifications.
What are the Similarities Between Elisa and Western Blot?
Elisa and Western blot are both techniques commonly used in the diagnosis of HIV. Despite some differences in their specific procedures, there are several similarities between the two methods:
- Immunodetection: Both Elisa and Western blot are based on immunodetection principles. They rely on the interaction between antibodies and proteins to identify and analyze specific molecules.
- Antibody-Protein Complex Formation: In both techniques, the formation of the antibody-protein complex is crucial for detection. Elisa uses the binding of antigens in the sample with immobilized antibodies on a solid surface, while Western blot involves the binding of antibodies with specific proteins transferred onto a solid membrane.
- Protein Analysis: Both Elisa and Western blot are used for the analysis of proteins. Elisa can detect and quantify various proteins, including HIV antibodies, while Western blotting allows for the separation and visualization of specific proteins from a mixture.
- Time-Consuming Techniques: Both Elisa and Western blotting can be time-consuming processes. They require several incubation steps, washing, and the addition of substrates or detection reagents, which may extend the overall testing time.
- Skilled Personnel: Both techniques require well-trained and skilled personnel to perform the procedures accurately. Proper handling, precise measurements, and interpretation of results are crucial for obtaining reliable and meaningful data.
While Elisa is often used as an initial screening test due to its high sensitivity, Western blotting is commonly employed as a confirmatory test for HIV diagnosis. Together, these techniques play important roles in the detection and confirmation of HIV infection, contributing to accurate diagnoses and appropriate patient care.
What is the Difference Between Elisa and Western Blot?
Characteristics of ELISA:
- Definition: ELISA is an immunosorbent assay that is used to detect antibodies or antigen in a sample.
- Estimation: Qualitative as well as quantitative.
- Gel Electrophoresis: Does not require the step of gel electrophoresis.
- Cost: Cost effective.
- Sensitivity: High sensitivity.
- Specificity: High specificity.
- Sample Requirements: Small sample volume.
- Complexity: Relatively less complex.
- Time Consuming: Moderate processing time.
- Equipment Needed: Standard laboratory equipment.
- Application: Initial screening test for HIV and other diseases.
- Skill Requirement: Requires skilled personnel for accurate interpretation and handling.
- Detection: Detection of antibodies or antigens in the sample.
Characteristics of Western Blot:
- Definition: Western blotting is an analytical technique that is used to separate and identify proteins from a mixture.
- Estimation: Qualitative and semi-quantitative.
- Gel Electrophoresis: Requires the step of gel electrophoresis.
- Cost: Costly.
- Sensitivity: Moderate to high sensitivity.
- Specificity: High specificity.
- Sample Requirements: Larger sample volume.
- Complexity: More complex.
- Time Consuming: Longer processing time.
- Equipment Needed: Specialized equipment required.
- Application: Confirmatory test for HIV and protein analysis.
- Skill Requirement: Requires skilled personnel for accurate interpretation and gel handling.
- Detection: Separation and identification of proteins.
ELISA vs Western Blot
| Characteristics | ELISA | Western Blot |
|---|---|---|
| Definition | ELISA is an immunosorbent assay that is used to detect antibodies or antigen in a sample. | Western blotting is an analytical technique that is used to separate and identify proteins from a mixture. |
| Estimation | Qualitative as well as quantitative | Qualitative and semi-quantitative |
| Gel Electrophoresis | Does not require the step of gel electrophoresis. | Requires the step of gel electrophoresis. |
| Cost | Cost effective | Costly |
| Sensitivity | High sensitivity | Moderate to high sensitivity |
| Specificity | High specificity | High specificity |
| Sample Requirements | Small sample volume | Larger sample volume |
| Complexity | Relatively less complex | More complex |
| Time Consuming | Moderate processing time | Longer processing time |
| Equipment Needed | Standard laboratory equipment | Specialized equipment required |
| Application | Initial screening test for HIV and other diseases | Confirmatory test for HIV and protein analysis |
| Skill Requirement | Requires skilled personnel for accurate interpretation and handling | Requires skilled personnel for accurate interpretation and gel handling |
| Detection | Detection of antibodies or antigens in the sample | Separation and identification of proteins |
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.