What is Electroporation?
Electroporation is a fascinating technique that temporarily makes cell membranes more permeable by applying brief electric pulses. This method, widely used in labs and medical settings, allows substances like DNA, drugs, or proteins to enter cells by creating tiny pores in the membrane. Imagine zapping cells with a controlled jolt of electricity—this disrupts the membrane’s structure just enough to let molecules slip inside without causing permanent damage. Once the electric field is removed, the membrane naturally reseals, leaving the cell intact. Over the years, this approach has become a go-to tool for tasks like genetic engineering, where inserting foreign DNA into bacteria or human cells is essential.
The roots of electroporation trace back to the 1960s, when researchers first noticed that electric fields could alter cell membranes. Early experiments focused on understanding how shocks affected things like muscle cells or blood tissues. By the 1980s, scientists had refined these observations into a practical method for gene transfer, coining the term “electroporation” as it entered mainstream lab use. Its appeal grew because it offered a physical alternative to chemical or viral delivery methods, which often had limitations in efficiency or safety. For instance, it became a game-changer for modifying plant cells, which have tough walls that other techniques struggled to penetrate.
Today, electroporation’s applications stretch far beyond basic research. Clinicians use it in gene therapy to treat genetic disorders, while oncologists explore its potential for delivering cancer drugs directly into tumors. Even the food industry has adopted the technique to kill harmful bacteria without heat, preserving flavor and nutrients. Its evolution from a curious lab phenomenon to a versatile tool underscores how blending physics with biology can unlock solutions across fields—all thanks to those brief, carefully timed pulses of electricity.
Principle of Electroporation
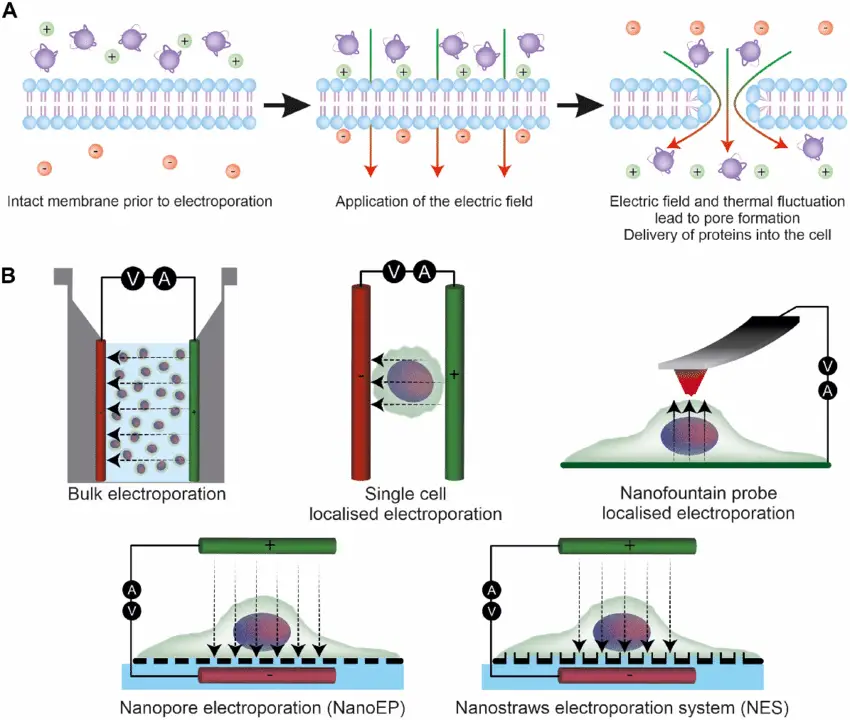
- Short, high-intensity electrical pulses applied to cells by electroporation momentarily disturb the lipid bilayer of the plasma membrane.
- The transmembrane potential created by the electrical pulse greatly surpasses the resting membrane potential of the cell, thereby causing phospholipids to rearrange.
- This reorganization creates transitory aqueous pores or flaws that let chemicals often unable of crossing the membrane into the cell.
- Factors include the size and duration of the electric pulse, the waveform used, and the conductivity of the surrounding medium determine the degree and efficiency of pore development.
- Usually resealing after the pulse, the cell membrane restores its barrier role; if the pulse parameters are too strong, the damage is permanent and may cause cell death.
- The mechanism is essentially based on electrophysiology and dielectric breakdown, in which the membrane, functioning as an insulator, loses its integrity when subjected to a critical electric field.
- The threshold and kinetics of pore development are further influenced by the shape and composition of the cell as well as external factors including temperature and ionic strength.
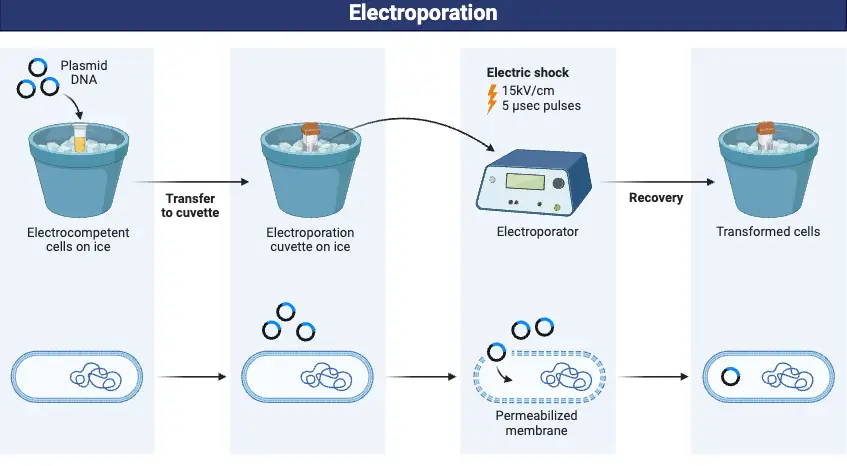
Steps of Electroporation
- Minimise ionic conductivity and avoid electrical arcing by preparing a cell suspension in an optimal, low-salt electroporation buffer.
- Move the cell suspension into a sterile electroporation cuvette such that the electrodes align correctly and the cells distribute uniformly.
- Choose the suitable voltage, pulse duration, number of pulses, and pulse interval depending on the particular cell type and chemical to be delivered, so setting and calibrating the electroporator.
- Apply the electrical pulse(s) to the cuvette; the electric field momentarily disturbs the phospholipid bilayer to create reversible nanopores in the cell membrane.
- Add pre-warmed recovery medium straight to the cuvette to aid in cell recovery and fast membrane pores’ resealing.
- Move the cells to an appropriate culture medium and incubate under ideal growth conditions to enable expression of the added molecules and restoration of normal cellular operations.
- Track cell survival and assess electroporation success by means of suitable assays, then analyzing the target molecule’s absorption or expression.
- Change the process settings as necessary for various cell types or molecular cargos to enhance effectiveness and reduce cellular damage.
Applications of Electroporation
- Both prokaryotic and eukaryotic cells can be genetically transformed by electroporation, so facilitating high-efficiency DNA absorption for cloning, recombinant protein manufacture, and the creation of transgenic cell lines.
- It is a fundamental part of DNA vaccination programs meant to generate strong immune responses and underpins gene therapy approaches by allowing therapeutic genes to be directly introduced into target tissues.
- Electroporation is used in electrochemotherapy in cancer treatment to increase the intracellular transport of chemotherapeutic drugs (e.g., bleomycin), hence improving local drug absorption and lowering of systemic side effects.
- In oncology, Irreversible electroporation (IRE) is a non-thermal ablation technique that selectively kills tumor cells while maintaining the extracellular matrix and vital components including blood arteries and nerves.
- In protein delivery studies, the method is crucial since it lets researchers introduce exogenous proteins into cells for study of intracellular signaling, protein interactions, and subcellular localization free from the need for viral vectors.
- In plant biotechnology, commonly known as electroporation, it helps plant cells with foreign DNA to undergo transformations resulting in genetically modified crops with improved features.
- Electroporation is a common technique for converting bacteria and yeast in microbiology; it produces high colony-forming units and is therefore absolutely essential for the synthesis of recombinant proteins and genetic research.
- Its use in tissue engineering and regenerative medicine, where tailored drug delivery and cell behavior modulation without generating heat harm are made possible by localized electroporation techniques, constitute emerging uses.
Advantages of Electroporation
- Often producing many more colony-forming units per microgram of DNA than chemical techniques, electroporation achieves great transformation efficiency.
- This non-viral approach lowers the toxicity and immunogenicity related with viral vectors.
- Broadly applicable to a wide spectrum of cell types, including bacteria, yeast, plant, and mammalian cells, the method is flexible for both research and clinical uses.
- Rapid and reasonably priced electroporation techniques require little specialized reagents and enable high-throughput uses by helping to
- It offers exact control over important parameters such voltage, pulse duration, and pulse frequency, thereby optimizing for particular cell types and payloads.
- Since it does not rely on transfection reagents, the technique reduces chemical toxicity, therefore retaining cell viability and repeatability.
- Under enabling targeted and localized delivery of therapeutic molecules, electroporation underlies advanced therapies such gene therapy and electrochemotherapy in clinical settings.
- It can be modified for both reversible uses—where cells restore normal function following brief pore formation—and irreversible uses such tissue ablation by irreversible electroporation, which protects extracellular structures while specifically causing cell death.
Disadvantages of Electroporation
- Electroporation calls for exact pulse parameter optimization, so inaccurate settings can cause too much membrane damage and lower cell viability.
- High voltage pulses might cause heat effects or irreversible pore creation, therefore compromising cellular processes and maybe resulting in cell death.
- Variations in cell size, membrane composition, and ambient circumstances often yield contradictory findings that need for careful protocol changes.
- The method limits its scalability and accessibility for major uses by depending on carefully regulated conditions and specialized, usually costly equipment.
- High salt concentrations or contaminants in the electroporation buffer might induce electrical arcing, therefore lowering efficiency and repeatability even further.
- Strong muscular contraction and maybe injury to surrounding tissues if not carefully controlled can be triggered in in vivo applications by the high electrical fields needed.
Common problems with electroporation
- Electrical arcing brought on by high salt concentrations or contaminants in the electroporation buffer destroys cells and lowers general efficiency.
- Inadequate optimization of pulse parameters (voltage, duration, and frequency) may result in either inadequate pore generation or irreversible membrane damage compromising cell viability.
- Different transfection outcomes over the cell population arise from unequal cell dispersion or clumping in the cuvette producing a non-uniform electric field.
- Air bubbles in the cuvette can cause unintentional arcing, which produces inconsistent results and higher cell mortality.
- Variability in cell type, size, and membrane composition calls for significant parameter changes, therefore challenging the standardizing of electroporation techniques over several experimental setups.
- Poor handling methods, including too strong pipetting or mixing, might mechanically harm cells, therefore lowering the transformation efficiency and cell survival.
- In in vivo treatments, problems with exact electrode placement and electric field dispersion in heterogeneous tissues can cause off-target harm and varying therapy effects.
- Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841-5. doi: 10.1002/j.1460-2075.1982.tb01257.x. PMID: 6329708; PMCID: PMC553119.
- http://link.springer.com/10.1007/BF01867861
- https://www.sciencedirect.com/science/article/abs/pii/016777998890025X?via%3Dihub
- https://www.sciencedirect.com/science/article/abs/pii/S0091679X08610432?via%3Dihub
- https://www.microbiologyresearch.org/content/journal/jgv/10.1099/0022-1317-70-12-3501
- https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2016.00099/full
- https://www.sciencedirect.com/science/article/abs/pii/016747819190162F?via%3Dihub
- https://www.technologynetworks.com/cell-science/articles/an-introduction-to-electroporation-a-tool-for-transfection-and-competent-cell-generation-363195
- https://en.wikipedia.org/wiki/Transformation_efficiency
- https://geneticeducation.co.in/electroporation-a-modern-gene-transfer-technique/