E. coli, short for Escherichia coli, is a versatile bacterium that can be found in a variety of environments, including certain foods, soil, and the intestines of animals, including humans. It belongs to the genus Escherichia and exhibits a wide diversity of strains. While many strains of E. coli are harmless and even beneficial, some can be extremely harmful, leading to serious health implications. One well-known example of a dangerous strain is E. coli 0157:H7.
The harmful strains of E. coli are responsible for causing food poisoning, leading to symptoms such as diarrhea and other digestive system problems. Moreover, these pathogenic strains can also give rise to additional health issues, including urinary tract infections, pneumonia, and respiratory illnesses.
In the human intestinal tract, E. coli serves a vital role in digestion and supports the absorption of essential vitamins from the food we consume. Additionally, it acts as a beneficial bacterium by inhibiting the growth and proliferation of other harmful species of bacteria that could otherwise lead to health problems.
There are several main categories of pathogenic E. coli, each with its own distinct characteristics and effects on the human body:
- Shiga toxin-producing E. coli (STEC): These strains produce Shiga toxins, which can cause severe food poisoning and lead to complications such as hemolytic uremic syndrome (HUS), a condition that affects the blood and kidneys.
- Enterotoxigenic E. coli (ETEC): This type of E. coli produces toxins that target the intestines, resulting in traveler’s diarrhea and other gastrointestinal issues.
- Enteropathogenic E. coli (EPEC): EPEC strains attach themselves to the intestinal lining, causing damage and disrupting normal absorption, leading to diarrhea and dehydration, particularly in infants.
- Enteroaggregative E. coli (EAEC): EAEC forms aggregations on the intestinal lining, resulting in persistent diarrhea, especially in children and travelers.
- Enteroinvasive E. coli (EIEC): Similar to Shigella bacteria, EIEC invades the intestinal lining, causing inflammation and symptoms similar to bacterial dysentery.
- Diffusely adherent E. coli (DAEC): DAEC adheres to the intestinal surface, potentially causing persistent diarrhea, especially in children in developing countries.
Due to the potential health risks posed by certain strains of E. coli, it is essential to handle and prepare food safely, maintain proper hygiene practices, and seek medical attention if symptoms of E. coli infection arise. Additionally, ongoing research and surveillance are crucial for understanding and combating the various strains of this bacterium.
Gram Stain of E. Coli
- The Gram stain is a widely used differential technique in microbiology that helps classify bacteria into two main groups: Gram-positive and Gram-negative. E. coli, being a Gram-negative bacterium, is one of the organisms that falls into the latter category.
- During the Gram staining process, bacterial cells are subjected to a series of staining steps. The primary stains used in this technique are crystal violet (a purple dye) and iodine. Following the application of these stains, the cells are then treated with an alcohol-based solution, called decolorization. Finally, a counterstain, usually safranin (a red dye), is applied.
- Gram-positive bacteria retain the crystal violet-iodine complex due to their thick peptidoglycan layer in the cell wall, which traps the dye. As a result, Gram-positive bacteria appear purple or blue under the microscope.
- On the other hand, Gram-negative bacteria like E. coli have a more complex cell wall structure. They possess an additional outer membrane composed of phospholipids and lipopolysaccharides (LPS). The presence of lipopolysaccharides on the outer membrane gives the cell wall an overall negative charge.
- When the decolorization step is performed in the Gram stain procedure, it washes away the crystal violet-iodine complex from the thin peptidoglycan layer of Gram-negative bacteria. As a consequence, these bacteria do not retain the primary stain and instead take up the counterstain, safranin. Therefore, when observed under a microscope, Gram-negative bacteria like E. coli appear red or pink.
- In summary, E. coli is classified as a Gram-negative bacterium because it does not retain the crystal violet stain during the Gram staining process. This is due to the presence of an outer membrane with lipopolysaccharides, which imparts a negative charge to its cell wall and causes the bacterium to take up the counterstain (safranin) instead, resulting in a red or pink appearance under the microscope.
E. Coli Under the Microscope Usign Gram Staining Method
E. coli microscopy is a technique used to examine samples for the presence of Escherichia coli bacteria. In this process, staining methods are employed, and the Gram stain is commonly utilized to distinguish between different types of bacteria in the sample, particularly differentiating between Gram-positive and Gram-negative bacteria.
The Gram stain is a differential staining technique, which means it provides more detailed information compared to simple stains like methylene blue. It involves the use of multiple stains to differentiate the cellular components of the bacteria in the sample.
In E. coli microscopy using the Gram stain, three different stains are used:
- Crystal violet: This is the primary stain used in the Gram staining process. It imparts a purple color to all bacterial cells in the sample.
- Iodine: After the application of crystal violet, iodine is used as a mordant. It forms a complex with the crystal violet dye, enhancing its adherence to the bacterial cells.
- Safranin: The final step in the Gram staining process involves the use of safranin as a counterstain. Safranin stains the cells that did not retain the crystal violet-iodine complex, distinguishing them from those that did.
Requirements for E. coli microscopy using the Gram stain method include a microscope glass slide, a collecting wire loop to obtain the bacterial sample, and a heater or burner for heat fixation of the sample on the slide. Additionally, distilled water is used for dilution and preparation purposes.
Materials Required
To conduct the experiment of E. coli microscopy using the Gram stain method, the following materials are required:
- Microscope glass slide: A clean and clear glass slide serves as the surface on which the bacterial sample will be placed and prepared for microscopic examination.
- A collecting wire loop: This is a simple tool made of metal, usually a loop or a wire with a handle, used to obtain a small amount of the bacterial sample. It is essential for transferring the bacteria onto the glass slide.
- A heater (Burner): The heater or burner is used for heat fixation of the bacterial sample on the glass slide. Heat fixation kills the bacteria, helps them adhere firmly to the slide, and enhances the staining process.
- Sample (E. Coli Culture): The sample contains the specific strain of Escherichia coli bacteria that needs to be examined. It can be a laboratory-cultured E. coli strain or a biological sample suspected to contain E. coli.
- Distilled water: Distilled water is required for preparing the bacterial sample and diluting the stains. Using distilled water ensures that no impurities or contaminants interfere with the staining process, which could affect the accuracy of the results.
- Gram stain (Crystal violet, Iodine stain, and Carbol Fuschin): The Gram stain kit contains the necessary dyes and reagents for the Gram staining process. It typically includes the following components:a. Crystal violet: This is the primary stain used in the Gram staining process. It imparts a purple color to all bacterial cells. b. Iodine stain: Iodine is used as a mordant, forming a complex with the crystal violet dye, enhancing its adherence to the bacterial cells. c. Carbol Fuschin: This is a counterstain used in some variations of the Gram stain method. It imparts a red or pink color to the cells that did not retain the crystal violet-iodine complex.
- Alcohol: Alcohol, usually ethyl alcohol or acetone, is a critical component in the Gram staining process. It acts as a decolorizing agent, removing the crystal violet-iodine complex from the bacterial cells, which allows the differentiation between Gram-positive and Gram-negative bacteria based on their cell wall characteristics.
Smear Preparation Procedure
The smear preparation procedure is a critical step in microbiology and is used to prepare bacterial samples for microscopic examination, staining, and identification. The steps involved in the smear preparation procedure are as follows:
- Heat the wire loop: Before starting the smear preparation, the wire loop used for scooping the sample should be sterilized to ensure there is no contamination. This is achieved by heating the loop over an open flame until it becomes red hot and then allowing it to cool. The cooling process is essential to avoid damaging the bacterial sample during the procedure.
- Scoop the sample onto a clean, sterile slide: Using the sterile and cooled wire loop, obtain a small amount of the bacterial sample, which could be from a broth culture or a plate culture. Place the sample onto a clean, sterile microscope glass slide.
- Spread the inoculum: Once the sample is on the slide, gently spread it in a circular or oval shape to cover an area of about a centimeter in diameter. This process is called spreading the inoculum and ensures an even distribution of bacterial cells on the slide.
- Saline solution for plate cultures: In the case of plate cultures, where bacterial colonies are grown on solid agar plates, it is necessary to add a drop of saline solution at the center of the slide. The saline solution helps in the suspension and dispersion of bacterial cells for further preparation.
- Form an emulsion: With the drop of saline solution on the slide, use another sterile wire loop (which has been heated and allowed to cool) to scoop a small amount of the bacterial sample from the culture. Stir the sample in the drop of water to form an emulsion, which is a uniform mixture of bacterial cells in the liquid medium.
By following these steps, the smear preparation procedure ensures that the bacterial sample is appropriately distributed and evenly spread on the microscope glass slide. This prepared smear is then ready for various staining techniques, such as the Gram stain, to help identify and classify the bacteria based on their staining characteristics. Additionally, the prepared smear is used for microscopic examination, aiding in the diagnosis of infections and the study of microbial morphology and arrangements. Proper and careful handling during the smear preparation is crucial to obtain accurate and reliable results in microbiological investigations.
Heat Fixing
Heat fixing is a critical step in the preparation of bacterial smears for microscopic examination. It involves the application of heat to the bacterial sample on a glass slide to kill the bacteria, firmly adhere them to the slide, and prevent them from getting washed away during the staining process. The heat fixing procedure typically includes the following steps:
- Air dry the slide: After spreading the bacterial sample on the glass slide and allowing it to mix with any necessary solutions (e.g., saline), the slide is left in open air to allow the smear to dry. Air drying ensures that excess moisture is removed from the slide before heat fixing.
- Pass the slide through the flame: Once the smear is air-dried, the slide is carefully held by its edge, and the side with the bacterial sample (smear side) is faced upward. The slide is then passed gently through the flame of a Bunsen burner or a gas flame a few times (usually 2 or 3 times) at a steady pace. The heat from the flame quickly kills the bacteria on the slide and promotes their attachment to the glass surface.
The purpose of heat fixing is to make the bacterial cells more heat-resistant, allowing them to withstand subsequent staining steps and microscopic examination without getting washed off or distorted. It also helps in coagulating the bacterial proteins, preserving their cellular structures, and preventing potential contamination.
However, during heat fixing, it is crucial to avoid overheating the slide. Excessive heat can damage the bacterial cells and alter their morphology, which can lead to inaccurate staining results and misinterpretation of microscopic observations. Therefore, heat fixing should be performed gently and for a brief duration to achieve the desired fixation without causing harm to the bacterial sample.
Proper heat fixing of bacterial smears is essential in microbiological studies, as it ensures that the bacteria are properly prepared for staining procedures and accurate identification under the microscope. By following the recommended steps and being cautious not to overheat the slide, researchers can obtain reliable and consistent results in their microscopic examinations and analyses.
Gram Staining
Gram staining is a widely used differential staining technique in microbiology to distinguish between two main types of bacteria: Gram-positive and Gram-negative. It involves a series of staining steps that help identify and classify bacteria based on the characteristics of their cell walls. The Gram staining procedure is as follows:
- Place the slide on a staining rack and flood the smear with crystal violet: The prepared bacterial smear on the glass slide is flooded with crystal violet, the primary stain, and allowed to sit for about one minute. Crystal violet imparts a purple color to all bacterial cells on the slide.
- Rinse gently with water: The slide is slightly tilted, and a gentle rinse with either tap or distilled water is performed. Rough washing should be avoided to prevent the removal of bacterial cells from the slide.
- Flood the sample with iodine: Iodine is used as a mordant to enhance the retention of the crystal violet dye by the bacterial cells. The bacterial smear is flooded with iodine and allowed to stand for about one minute.
- Rinse gently with water: Similar to the previous step, the slide is slightly tilted, and a gentle rinse with water is performed to remove excess iodine.
- Decolorize using 95% ethyl alcohol/acetone: The critical step in the Gram staining process is the decolorization. The slide is tilted, and 95% ethyl alcohol or acetone is applied as drops onto the smear for about 8 seconds. This step differentiates between Gram-positive and Gram-negative bacteria based on their cell wall composition.
- Rinse gently with water: The slide is rinsed gently with water to remove the decolorizer without disturbing the remaining stained bacterial cells.
- Blot the slide dry: Excess water is blotted off the slide using blotting paper or a paper towel.
- Place the slide on the microscope and view the sample: The prepared slide is placed on a microscope stage, and the bacterial cells are observed under the microscope. Gram-negative bacteria, such as E. coli, will appear pink or red in color, while Gram-positive bacteria will retain the purple color of the crystal violet.
The Gram staining technique allows microbiologists to quickly differentiate between Gram-positive and Gram-negative bacteria, providing valuable information about their cell wall structures. This information is useful for the initial identification and classification of bacterial samples, aiding in the diagnosis of infections and guiding appropriate treatment decisions.
Precautions for Gram Staining of E. Coli
When performing the Gram stain of E. coli, it is essential to take the following precautions to obtain accurate and reliable results:
- Use a fresh culture of E. coli: The bacterial culture used for the Gram stain should be less than 24 hours old. Older cultures may not provide accurate staining results.
- Prepare the slide carefully: Ensure that the bacterial smear on the slide is one cell thick and evenly spread. A thick smear will hinder proper stain penetration, while a thin smear may not allow clear visualization of the cells.
- Fix the slide properly: Use appropriate fixation methods such as heat fixation for 10 seconds or methanol fixation for 1 minute. Proper fixation helps the bacterial cells adhere to the slide and prevents them from being washed away during the staining process.
- Use correct staining reagents: Ensure that the correct concentrations of crystal violet, iodine, and decolorizer are used. Proper staining reagents are crucial for achieving accurate Gram staining results.
- Rinse the slide thoroughly: Thoroughly rinse off all staining reagents from the slide before counterstaining. Incomplete rinsing can affect the final staining outcome.
- Use the correct counterstaining reagent: Safranin is the commonly used counterstaining reagent for Gram staining. It helps visualize Gram-negative bacteria as they appear pink or red under the microscope.
- Observe the slide under the microscope: For proper interpretation of the Gram stain, use a bright-field microscope with a 10x or 40x objective lens. Observe the stained bacteria under oil immersion for higher magnification and clearer visualization.
Additional precautions when working with E. coli:
- E. coli is a pathogenic bacterium, so always take necessary precautions to prevent the spread of infection. Wear gloves and a lab coat when handling E. coli cultures.
- Properly dispose of all waste materials, including E. coli cultures, following biosafety regulations and guidelines.
Additional tips for performing a Gram stain of E. coli:
- Ensure the microscope is properly calibrated and focused before observing the slide.
- Observe the stained bacteria for several minutes to determine if they are Gram-positive (appear purple) or Gram-negative (appear pink/red).
- If the bacteria are not clearly visible, consider using a counterstain or adjusting the microscope settings to enhance visibility.
By following these precautions and tips, researchers can obtain accurate and reliable Gram stain results for E. coli, aiding in its identification and classification in microbiological studies.
Observation
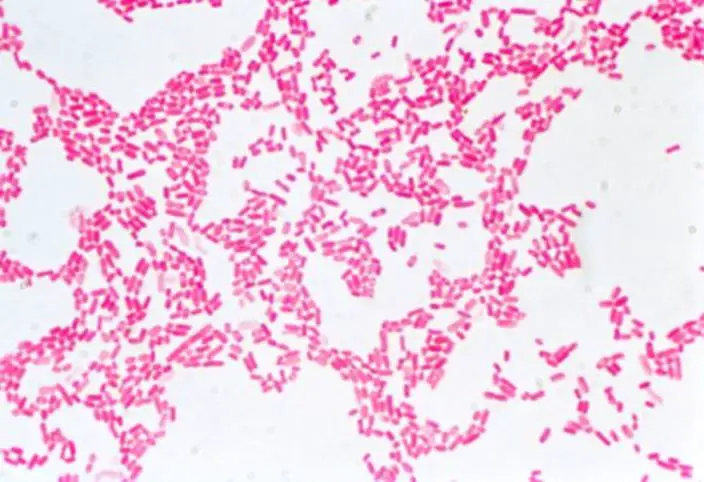
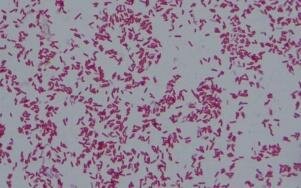
Hanging Drop Method for Observation of E. Coli Under the Microscope
The hanging drop method is a valuable technique used in microbiology to observe small, living, and unstained organisms, particularly motile bacteria such as Ps. fluorescens and E. coli. This method allows researchers to study the behavior and motility of these microorganisms in their natural state, providing valuable insights into their characteristics and behavior.
Material Required
The “Materials Required” for the hanging drop method in microbiology include the following:
- Clean glass slides: High-quality, clean, and transparent glass slides are necessary for preparing the hanging drop slide. These slides provide a smooth surface for mounting the bacterial sample.
- Paraffin wax: Paraffin wax is used to create a small ring or barrier around the drop of culture medium or saline solution on the glass slide. The paraffin wax ring acts as a spacer to prevent the coverslip from pressing down directly on the bacterial sample, allowing a small gap for the hanging drop preparation.
- Wire loop: A sterile wire loop is used to inoculate the drop of culture medium or saline solution with a small amount of the motile bacterial culture. The wire loop must be sterilized before use to avoid contamination.
- A cover slip: A clean and thin cover slip, usually made of glass, is placed over the drop of bacterial culture on the glass slide. The coverslip is pressed down gently to form a seal and create the hanging drop preparation.
- Heater (Bunsen burner): A Bunsen burner or a similar heat source is used for heat fixation of the bacterial sample on the glass slide. Heat fixation kills the bacteria, secures them to the slide, and enhances their visualization under the microscope.
- A young broth culture of motile bacteria: For the hanging drop method, a young broth culture of motile bacteria is required. The bacterial culture should preferably be within 24 hours of growth to ensure that the organisms are actively motile. Motile bacteria, such as Ps. fluorescens and E. coli, are particularly suitable for this technique, as their movement can be observed effectively using the hanging drop method.
Procedure
The procedure for the hanging drop method in microbiology involves the following steps:
- Apply a paraffin ring on a clean glass slide: To create a spacer for the hanging drop preparation, a paraffin ring is applied on a clean glass slide. An alternative to the paraffin ring is using an adhesive tape ring.
- Dab Vaseline on the corners of a cover slip: Vaseline is applied to the corners of a cover slip to create a seal between the cover slip and the slide, ensuring that the hanging drop preparation stays in place during observation.
- Place a loopful of the broth on the center of the glass cover slip: Using a sterilized wire loop, a small amount of the broth culture containing the motile bacteria is carefully placed on the center of a glass cover slip.
- Turn the glass slide over the sample on the cover slip: The glass slide is carefully inverted and placed over the cover slip with the bacterial sample. The sample should now lie between the slide and the cover slip, creating the hanging drop preparation.
- Place the slide on the microscope for viewing: The prepared hanging drop slide is placed on the microscope stage for observation. The cover slip should be on top, allowing clear visualization of the motile bacteria.
- Start with the lowest power and view the specimen: The microscope is initially set to the lowest magnification (lowest power) to locate the bacterial sample on the hanging drop slide. Once located, the magnification can be increased to observe the motility and behavior of the bacteria more closely.
To determine if the bacteria are genuinely motile, it is essential to observe their movement in different directions and changes in position. This differentiation helps distinguish true bacterial motility from the random movement known as Brownian motion.
Precautions for Hanging Drop Method
When performing the hanging drop method to observe the motility of bacteria under the microscope, it is important to take certain precautions to ensure accurate and reliable results:
- Use clean microscope slide and cover slip: Any dirt, dust, or debris on the slide or cover slip can hinder clear observation of the bacteria. Make sure both the slide and cover slip are clean and free from contaminants.
- Use a small amount of liquid: Adding too much liquid to the hanging drop preparation can lead to difficulties in focusing the microscope and may cause the bacteria to be washed away. Use just enough liquid for the observation.
- Place the slide in a humid environment: To prevent the liquid in the hanging drop from evaporating too quickly, place the slide in a humid environment or use a moist chamber during observation.
- Observe the bacteria quickly: Bacteria in the hanging drop can start to die if they are not kept moist. To obtain accurate results, observe the bacteria promptly after preparing the hanging drop slide.
- Place the slide on a level surface: Ensure that the slide is placed on a level surface to evenly distribute the hanging drop of liquid. An uneven distribution can affect the observation of the bacteria.
- Avoid touching the coverslip with your fingers: Touching the coverslip with your fingers can introduce oil or contaminants that may interfere with the observation of the bacteria. Handle the coverslip by its edges or use forceps.
- Observe under low power magnification first: Start the observation under low power magnification to get a general overview of the bacteria and their motility patterns before moving on to higher magnification.
- Be patient: Bacterial motility may take some time to become visible under the microscope. Be patient and give the bacteria enough time to exhibit their movement.
By following these precautions, researchers can ensure the hanging drop method provides accurate and reliable information about the motility and behavior of the bacteria being studied. Proper handling and observation techniques are essential to obtain valuable insights into the characteristics of the microorganisms under investigation.
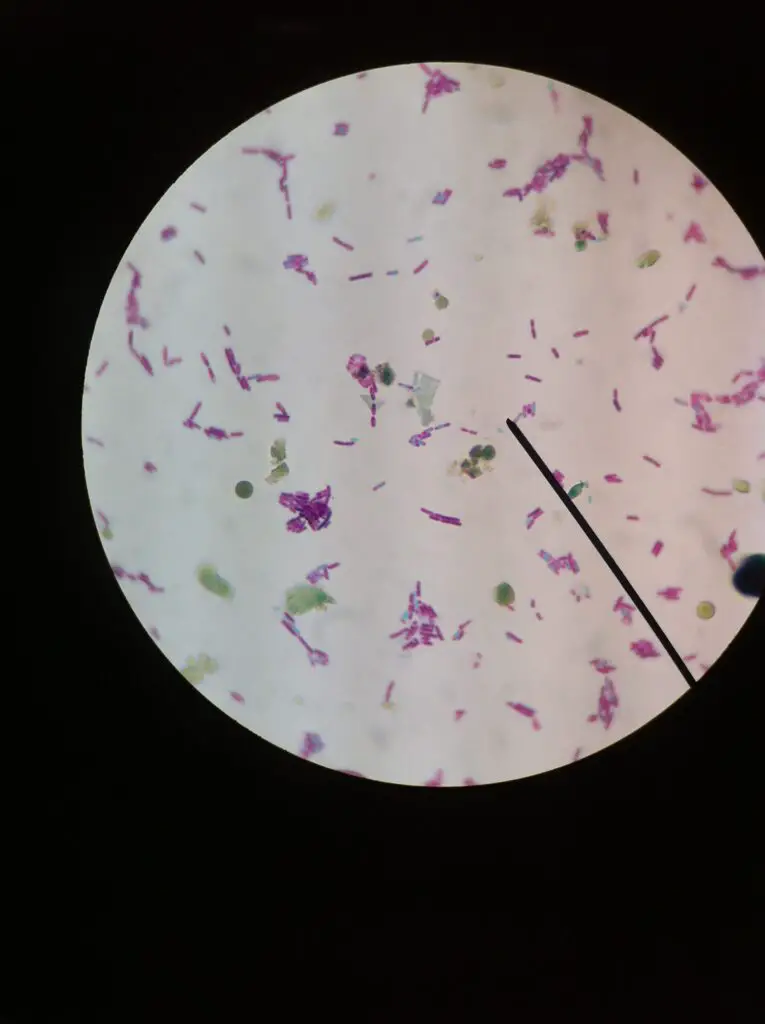
Observation of E. Coli Under the Microscope
FAQ
What does E. coli look like under the microscope?
E. coli appears as small, rod-shaped bacteria when viewed under the microscope. They typically have a single flagellum or multiple flagella, which contributes to their motility.
How do you prepare an E. coli sample for microscopy?
An E. coli sample can be prepared for microscopy using the smear preparation technique. A small amount of the bacterial culture is spread thinly and evenly on a glass slide, followed by heat fixation to secure the bacteria to the slide.
What magnification is best for observing E. coli under the microscope?
E. coli can be observed at various magnifications. Initially, lower magnifications (e.g., 100x) are used to locate the bacteria, and higher magnifications (e.g., 1000x) are employed to observe their details and motility.
How can you distinguish E. coli from other bacteria under the microscope?
E. coli can be distinguished based on its rod-shaped morphology and motility, which can be observed under the microscope. Additionally, specific staining techniques like the Gram stain can further aid in the identification of E. coli based on its Gram-negative properties.
What color does E. coli appear under the microscope after Gram staining?
E. coli appears pink or red under the microscope after Gram staining, indicating that it is a Gram-negative bacterium.
What type of motility does E. coli exhibit under the microscope?
E. coli exhibits peritrichous motility, meaning it possesses multiple flagella distributed over its entire surface, allowing it to move in various directions.
Can E. coli be observed moving actively under the microscope?
Yes, E. coli is motile and can be observed actively moving under the microscope. Its peritrichous flagella enable it to exhibit rapid and agile movements.
What are the typical characteristics of E. coli colonies when viewed under the microscope?
E. coli colonies are smooth, moist, and slightly raised when viewed under the microscope. Individual bacterial cells from these colonies display their characteristic rod shape.
Can E. coli be easily identified under the microscope alone?
While the microscopy provides valuable information, the identification of E. coli may require additional tests, such as biochemical tests, antigen testing, or molecular techniques like PCR.
Is E. coli harmful to observe under the microscope?
E. coli strains that are typically used in laboratories are not harmful to observe under the microscope. However, some pathogenic strains of E. coli can cause illnesses, and proper safety precautions should be taken when working with potentially harmful strains.