Osmosis refers to the movement of water molecules through semi-permeable membranes from high to low water potential. This semi-permeable membrane does not allow for solute particles to pass through it, but solvent particles (water molecules), can move across it. Tonicity can be described as the degree of the osmotic pressure gradient. There are three states. There are three types of tonicity: hypertonic (isotonic), hypotonic (hypotonic), and one for each state. Hypotonic solution, which is the lowest solute concentration, is preferred over the other two. Hypertonic solution has a higher solute concentration. This process is driven by the solvent concentration gradient between the two solutions. Because of the unbalanced osmotic pressure, the net movement of the solvent between hypotonic and hypertonic solvents takes place.
Hypotonic Solution
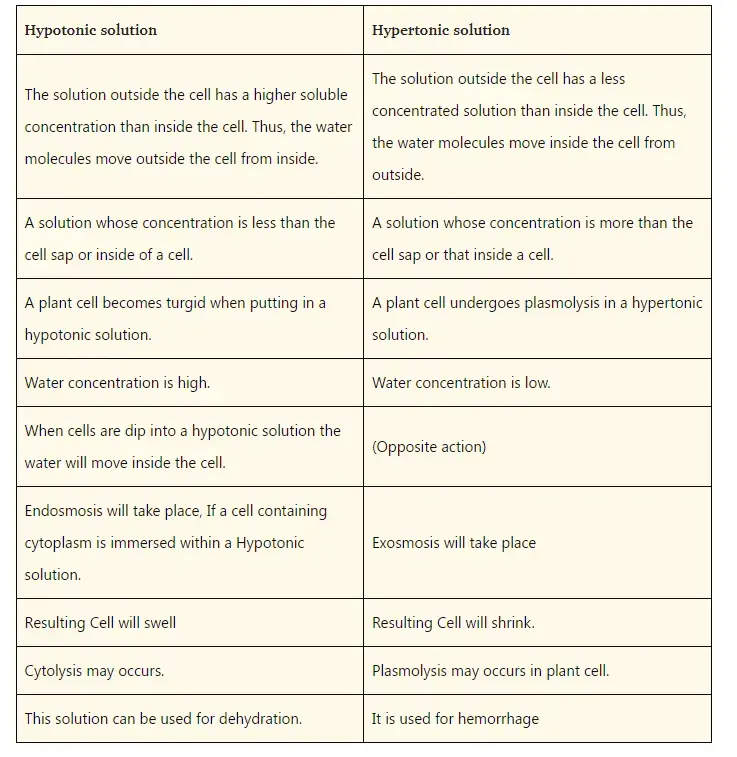
Hypotonic solutions are those that have a lower solute concentration than the inside of a cell. This solution has a very low osmotic potential compared to other solutions. Hypotonic solutions can cause water molecules to move within cells due to their osmotic potential.
Cell swelling would be caused by the constant diffusion of water molecules inside cells. It may also cause cell swelling (or rupture). Plant cells don’t burst because they have a rigid wall.
Hypertonic Solution
Hypertonic solutions contain a higher concentration of solutes in the solution than the inside of a cell. The solution will cause water molecules to escape from the cell when it is placed in hypertonic solutions. The water moving from the cell to the exterior causes the cell to become distorted and wrinkled. This is known as ‘crenation of the cell’. The flexible plasma membrane in plant cells pulls away from the cell wall but is still attached to it at certain points by the effect of crenation. This results in the condition known as ‘plasmolysis’.
Difference Between Hypotonic vs Hypertonic
Hypertonic solutions have the solute being greater than the solvent. The solute could be the table sugar, while the solvent would be the water. Hypotonic is the opposite. The solute is smaller but the solvent is larger.
These concepts can be applied in the real world to the body and can be used to treat dehydration, hypovolemia and hypervolemia as well as other fluid and electrolyte imbalances. Nurses and doctors are able to quickly intervene in the care of patients who require immediate treatment by understanding the concepts of hypotonicity or hypertonicity. These fluids can be administered intravenously.
It can also be used to treat cerebral hemorhage as hypertonic solution. It has a calming effect on the body, particularly in the intracellular as well as extracellular spaces. By allowing fluids to flow out of cells, it shrinks them. Solutions with higher solutes attract water. The intravascular space is the place where blood cells are confide. One patient may have a cerebral hemorhage. This is a condition where too much blood is leaking, causing hypovolemia. A hypertonic solution can be administered to the patient. This will cause the water in the blood cells to drain out, restoring fluid circulation. Examples of intravenous hypertonic solution are D5LR or D5.45 Na Cl.
Hypotonic solutions can be used to treat hypernatremia or dehydration. Hypotonic solutions cause swelling by allowing the cells to absorb water. Hypotonic solutions have a lower solute, which means that water will move from the solution to the cell. Hypotonic solutions can help a patient suffering from dehydration (low water content) by allowing water to move back into the cells. Hypotonic intravenous solutions include 0.45 Na Cl or 0.25 Na Cl.
This is crucial information for medical professionals who need to intervene in cases of severe hypovolemia, severe dehydration, or hemorhage. Medical practitioners will be able to act in a timely manner to save their patients’ lives if they are able to master this concept.