What is Lane-Eynon Method?
- Lane-Eynon Method is a titration procedure used for estimation of reducing sugars (like glucose, fructose etc.).
- The method based on reduction of copper(II) ions to copper(I) oxide in alkaline medium using Fehling’s solution.
- During titration, reducing sugar acts as reducing agent, and blue Cu²⁺ solution gradually change to reddish precipitate of Cu₂O.
- Indicator methylene blue is added, it gets reduced last after all Cu²⁺ has reacted. When blue color disappear – end-point reached.
- Usually Fehling A (CuSO₄ solution) and Fehling B (alkaline tartrate solution) are mixed in equal volume just before titration.
- Reaction carried out under boiling condition, and the volume of sugar solution required to decolorize indicator recorded.
- Standardization often done using invert sugar or glucose standard, result expressed as percentage of reducing sugar in sample.
- The method mostly applied for sugar analysis in food, fruit juice, honey, etc., but not suitable when non-reducing sugars present.
- Accuracy depend by proper boiling time, concentration of reagent, and correct observation of end point – these variation cause small error sometimes.
Principle of Lane-Eynon Method
The method based on reduction of cupric ions (Cu²⁺) to cuprous oxide (Cu₂O) by the reducing sugars in alkaline condition.
In the reaction, CuSO₄ from Fehling A and alkaline tartrate from Fehling B react to form a deep blue cupric–tartrate complex, which remain stable in hot solution for short time.
When reducing sugar solution added and boiled, it donates electrons to the cupric ions, and they reduced to cuprous oxide, which precipitate as red or orange solid.
The end point of titration determined when all Cu²⁺ ions converted, and the last trace of blue color of methylene blue indicator disappears.
The principle rely on stoichiometric relation between the quantity of reducing sugar and amount of copper(II) reduced.
Reaction generally written as:
RCHO + 2Cu(OH)₂ → RCOOH + Cu₂O↓ + 2H₂O
Thus, the more sugar present, the more Cu₂O formed – and this amount can be measured indirectly by the titration volume.
This chemical reduction process is quantitative, assuming complete reaction under boiling and alkaline condition.
Requirements for Lane-Eynon Method
- Fehling’s Solution A – aqueous CuSO₄ solution (standardized usually with glucose).
- Fehling’s Solution B – mixture of alkaline sodium potassium tartrate (Rochelle salt) and NaOH.
- Reducing sugar solution (sample solution to be tested – like glucose, fructose etc.).
- Methylene blue indicator, used for detecting end-point (color change blue → colorless).
- Standard glucose solution, used for standardization of Fehling’s solutions.
- Burette, pipette, and conical flask, for titration setup – glass must be clean and dry for accuracy.
- Measuring cylinder / volumetric flask, for preparing and diluting solutions to desired volume.
- Boiling water bath or burner, to maintain solution at boiling temperature (~100 °C or 100°C both ok).
- Distilled water, for all preparations to avoid ions that may interfere with copper reduction.
- Clamp stand and wire gauze, used for heating support of flask during boiling.
- White tile sometimes used for better visibility of end-point discoloration.
- Safety items like gloves / lab coat / goggles also needed as alkaline Fehling is corrosive.
Procedure
- Fehling’s A and Fehling’s B were mixed freshly in equal volumes just before titration.
- About 10 mL of the mixed Fehling’s was pipetted into a 250 mL conical flask, and distilled water (≈ 10 mL) was added.
- The flask was heated to gentle boiling, the solution was kept boiling (so reaction kinetics were maintained).
- The reducing sugar sample was placed in the burette and it was delivered slowly while the Fehling’s was boiling.
- Constant swirling was ensured, and drops of sugar solution were added until the blue color started to fade.
- Methylene blue indicator (2–3 drops) was added when near end-point, and the titration was continued dropwise.
- The end-point was recorded when blue color disappeared completely and reddish Cu₂O precipitate remained.
- Volume of sample used was noted, titration was repeated at least three times, and concordant readings were selected.
- Standardization was done with standard glucose solution, and factor was applied to calculate % reducing sugar.
- Care was taken that overheating was avoided — overheating may decompose the cupric tartrate complex, and error will be introduced, prevail was attempted to be avoided.
- Glassware cleanliness was ensured, distilled water used, and safety gear (gloves/goggles) was recommended.
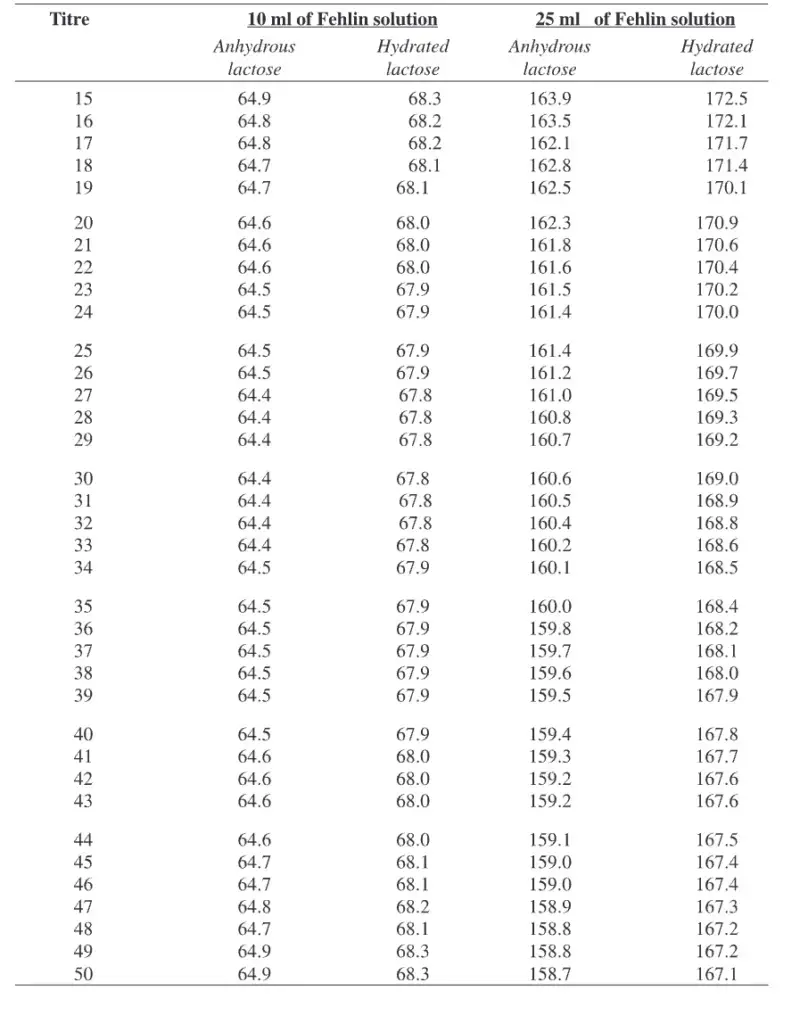
Results of Lane-Eynon Method
The result of this method expressed as percentage of lactose (or other reducing sugar) present in the sample.
Calculation depend by relation between Factor and Titre, obtained from titration reading.
Formula used: Lactose (%) = (Factor / Titre) × 100
Factor = predetermined constant, showing how much lactose is equal to the Fehling’s solution reduced completely. It derived using standard lactose solution.
Titre = volume of the sample (in mL) used to reach end-point – that’s when methylene blue color disappears.
After both values known, the division of Factor by Titre, then multiplied × 100, gives the percentage lactose in sample.
Example – if Factor = 0.5 mg/mL and Titre = 25 mL,
then Lactose (%) = (0.5 / 25) × 100 = 2 %.
That mean the sample contain 2 % lactose (by weight or volume, depend on sample prep).
Result reliability depend on accurate standardization of Fehling’s solutions and correct titration endpoint – even small mistakes cause deviation.
Precision must be maintained; mis-reading or improper boiling may prevail the error, causing wrong percent.
Results usually recorded in table form with average titre, factor used, and % lactose calculated for clarity.
Factor for Fehlin solution to be used in correction with the Lane-Eynon general volumetric method.
Uses of Lane-Eynon Method
- The Lane–Eynon Method is mainly used for determining reducing sugars in many food and beverage samples.
- It is widely applied in dairy industry to estimate lactose content in milk and milk products.
- Used for quantitative analysis of sugars like glucose, fructose, and maltose in jams, honey, syrups, and fruit juices.
- Applied in confectionery testing to check sugar inversion during processing or storage.
- Used in pharmaceutical industry for sugar estimation in syrups and tonics – especially where glucose is present.
- Sometimes employed in fermentation studies, for monitoring residual sugar concentration in fermentation broth.
- Helpful in quality control laboratories for verifying sugar levels in processed food.
- Used in research for calibration of other sugar–determination methods, since it gives comparative reducing sugar value.
- The method also used in teaching laboratories – for demonstrating redox reaction between Cu²⁺ and reducing sugars.
- Less useful when non-reducing sugars present, as they don’t react directly, but hydrolysis can be done first to make them measurable.
- Small procedural modification allow its use in lactose estimation in milk whey etc., though overheating may prevail small experimental errors.
Advantages of Lane-Eynon Method
- The method simple and quick, can be done without complex instrument.
- Fehling’s reagents easily prepared from common laboratory chemicals.
- The end-point easily observed by disappearance of blue color – visual and clear.
- The procedure gives fairly accurate result when done carefully and reagents standardized properly.
- Applicable to many sugar-containing samples — milk, juice, honey, etc.
- Can be used for routine sugar estimation in industrial and academic labs.
- Method is quantitative, allowing exact % of reducing sugars to be determined.
- Cost of analysis is low, no expensive apparatus required.
- The reaction principle (reduction of Cu²⁺ to Cu₂O) is well understood, so calculation is straightforward.
- It also useful for teaching purpose, demonstrating oxidation-reduction reaction visually.
- Few modifications make it suitable for estimation of lactose, glucose, and invert sugar, hence quite versatile.
- Minor procedural error or overheating may prevail small deviation, but method still robust and repeatable.
Disadvantages of Lane-Eynon Method
- The method time-consuming when many samples handled, because each titration need boiling.
- The end-point judged by eye, so subjective error may occur if color change not seen clearly.
- Non-reducing sugars like sucrose cannot be measured directly – they must be hydrolyzed first.
- Overheating of Fehling’s mixture may decompose the Cu-tartrate complex, giving false readings.
- Presence of impurities or other reducing agents (like ascorbic acid) interfere and make error in result.
- The method not suitable for colored or turbid samples, because color hides the indicator change.
- Accuracy depends strongly on standardization of Fehling’s solution, which may change on storage.
- Difficult to automate; only manual titration can be done, so slow for industrial routine.
- The test requires boiling condition, so unsafe handling if done carelessly (splashing, burns possible).
- Slight change in boiling time or volume of solution can prevail variation in data, giving inconsistent % sugar.
- Result expression depend on calculation factor – if that factor wrong, whole data become unreliable.
Precautions
- Fehling’s A and Fehling’s B must always be mixed fresh just before titration, old mixture may decompose.
- The titration flask should be clean and dry; any residue or drop of previous solution affect the reaction.
- Boiling should be gentle, not violent – too strong heating can cause decomposition of Cu-tartrate complex.
- Methylene blue indicator must be added near the end-point only, not at start; early addition may reduce too soon.
- The titration should be done rapidly once boiling begins; long boiling gives oxidation error.
- Always maintain same boiling temperature, irregular heating may prevail false readings.
- Use distilled water for all solution preparation; tap water may contain ions which react with copper.
- While heating, the flask must be supported on wire gauze or water bath, not directly on flame (to avoid bumping or crack).
- End-point color change should be observed carefully under good light, because the last trace of blue may persist faintly.
- Do not allow the precipitate of Cu₂O to settle before finishing titration – swirl continuously.
- The Fehling’s reagent should be standardized often using standard glucose or lactose solution.
- Use proper pipette and burette calibration; any parallax or mis-reading directly affect result.
- All glassware rinsed with the solution to be used – this keeps concentration consistent.
- Avoid contamination with reducing agents (like ascorbic acid or aldehyde fumes), which can falsely increase sugar result.
- Safety: wear gloves, goggles, and lab coat, as the alkaline Fehling’s is corrosive and can irritate skin.
- Lane, J. H., & Eynon, L. (1923). Determination of reducing sugars by Fehling’s solution with methylene blue indicator. Journal of the Society of Chemical Industry, 42(1), 32–37.
- Pearson, D. (1976). The Chemical Analysis of Foods (7th ed.). Churchill Livingstone.
- Ranganna, S. (1986). Handbook of Analysis and Quality Control for Fruit and Vegetable Products. Tata McGraw-Hill.