A colorimeter is a scientific instrument used to measure the absorbance of light by a colored solution to determine the concentration of solutes. Based on the Beer-Lambert law, the principle of colorimeter explains how light intensity changes as it passes through a solution. The instrument mainly consists of essential parts of colorimeter like a light source, filter, cuvette, and detector, which together help in measuring optical density precisely.
In laboratories, the procedure of colorimeter is simple, making it a reliable tool for quantitative analysis in biology, chemistry, and environmental science. The applications of colorimeter are wide — from clinical diagnosis and water testing to food analysis and research experiments. Overall, a colorimeter plays a crucial role in modern analytical techniques for accurate and quick color-based estimations.
What is a Colorimeter?
- Colorimeter can define as a scientific instrument used for measuring the absorbance of particular wavelengths of light by a specific solution.
- It based on principle of Beer-Lambert law, which tells that absorbance is directly proportional to concentration of the solution.
- In simple way, it measures how much light is absorbed (or transmitted) by colored compounds in solution.
- Mostly used for quantitative estimation of substances like proteins, glucose, etc., in biology and chemistry laboratories.
- The readings are taken by comparing intensity of light before and after passing through the solution.
- A colorimeter consists of a light source, filter, cuvette holder, and a photoelectric detector.
- The light is passed through a filter that selects specific wavelength, then through the test solution, finally detected and converted into electrical signal.
- It’s simple, sturdy, and handy equipment used widely for quick color-based analysis.
- Operation of colorimeter is mostly manual but some modern types are automatic/digital which provide fast results.
- It is found very useful in fields like clinical diagnostics, water analysis, food testing etc.
- The colorimeter is important tool for determining unknown concentration of solute in a solution with good accuracy and less time.
- It allows rapid chemical analysis without complex instrumentation.
- It’s cheap and easily available equipment, so it’s commonly used in teaching and research laboratories.
- Accurate color measurements help to understand purity, contamination, or reaction progress of substances.
- Without it, many biochemical experiments can’t done effectively because visual color observation are not reliable.
- The development of colorimeter goes back to 19th century when early optical devices were designed for color comparison.
- The first practical photoelectric colorimeter was developed around 1930s, after that many improved versions were made.
- Earlier, comparison was done by eye using colored glass standards but later replaced by electronic detectors.
- Over time, technology got advanced and modern digital colorimeters were introduced, which they gave more accuracy and stability.
- Thus, the colorimeter has evolved from a simple visual tool to a modern photometric instrument widely used in science and industry.
Colorimeter Definition
A colorimeter is a scientific instrument used to measure the absorbance of specific wavelengths of light by a solution, aiding in determining the concentration of a solute within that solution based on the Beer-Lambert law.
Principle of Colorimeter – How does a colorimeter work?
- The working principle of a colorimeter is based on the Beer-Lambert law (or Beer’s law + Lambert’s law) which states that light absorption by a solution is directly proportional to the solute concentration and the path length of light.
- A beam of monochromatic (or near-monochromatic) light of intensity I₀ is passed through the solution, and a part is absorbed (Iₐ) and the rest transmitted (Iₜ) so I₀ = Iₐ + Iₜ (reflection is minimized by using identical cuvettes) .
- The amount of light transmitted (or conversely absorbed) is measured by a detector, and then the absorbance A is given by A = log₁₀(I₀ / Iₜ) or A ∝ c l (where c is concentration, l is path length) .
- In practice the instrument is calibrated with standards of known concentration so that unknown sample’s concentration can be read from a calibration curve, and thus the measurement is more reliable.
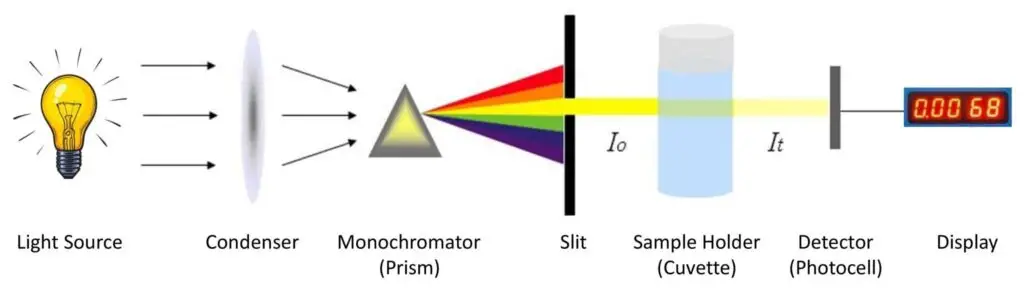
- Inside the colorimeter a light source (for example a tungsten lamp or LED) emits white light, then a filter selects a specific wavelength (complementary to the colour of solution) and then the light passes through the cuvette containing solution and reaches a photo-detector which converts the light intensity into electrical signal.
- More concentrated solutions absorb more light and allow less transmitted light to reach the detector, so the measured signal changes accordingly; the instrument uses that change to calculate the concentration and often displays either % transmittance or absorbance.
- Some limitations occur: for very high absorbances the linear relation may fail, or if the wrong wavelength is used or cuvette is scratched then errors creep in.

Diagram of Colorimeter
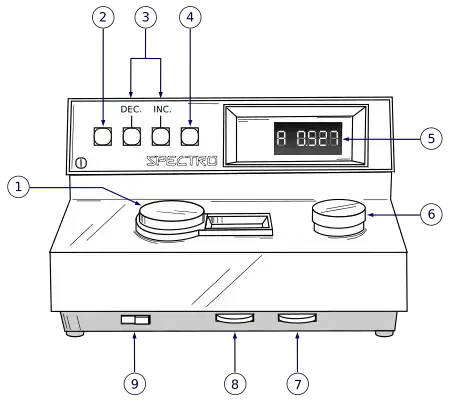
Mathematical Equation of Colorimeter
The basic relation is A = ε c l, where A (absorbance) is measured by the colorimeter and ε (molar absorptivity or extinction coefficient) × c (concentration) × l (path-length) are the factors.
According to Beer-Lambert law the amount of light absorbed is directly proportional to concentration and path length, so A ∝ c l and then equality is given by A = ε c l.
The relation between incident intensity I₀ and transmitted intensity Iₜ is given by A = log₁₀(I₀ / Iₜ); this is used by the instrument (with reflection ignored) to compute absorbance.
In practice the equation is manipulated: when ε and l are constant then c = A / (ε l) which allows unknown concentration to be found from measured absorbance.
Limitations must be noted: at high concentrations or with scattering the linearity fails and the equation A = ε c l may not hold perfectly.
Parts of Colorimeter/ Components of Colorimeter
The Important Parts of Colorimeter are;
- Light Source – The light source provides energy of enough intensity to cover full visible spectrum (about 380–780 nm). Usually Tungsten lamps are used for visible/near-infrared region, while Halogen-Deuterium lamp suitable for UV range (200–900 nm). It’s important that light is steady and uniform else error may prevail.
- Slit – A narrow slit is provided for reducing stray or unwanted light, only allowing a thin beam to pass. This helps to make beam parallel and accurate for passing by next optical part.
- Condensing Lens – The lens collects and focuses light; after passing the slit, a parallel beam of light emerges by condensing lens and it directs the light towards the monochromator/filter.
- Monochromator – The function of monochromator is to isolate monochromatic light from polychromatic one, filtering only a specific wavelength useful for analysis. Three main types are used– Prism, Grating, and Glass.
- Prism – causes refraction of light when it passes from one medium to another, separating wavelength by angle.
- Glass – it selectively transmits specific wavelength range and absorbs others.
- Grating – made usually of graphite or ruled surface which separates the light into several wavelengths by diffraction.
- Cuvette (Sample Cell) – After filtering, the monochromatic light passes through colored solution placed inside cuvette. The cuvettes come in shapes like square, rectangular or round, generally of fixed path length 1 cm. Based on material, three main types are used:
- Glass cuvette – cheap, used for visible light, absorbs below 340 nm.
- Quartz cuvette – used for UV and visible both, more durable.
- Plastic cuvette – inexpensive, but easily scratched and shorter lifespan.
- Photocell / Photodetector – This component detects transmitted light intensity. The photocell converts light energy into electrical energy which corresponds to absorbance value.
- Galvanometer (or Display Unit) – The electrical signal from photocell is measured by a galvanometer or digital meter. It shows reading in Optical Density (OD) or % Transmittance, sometimes both are displayed for easy comparison.
- Aperture / Housing parts – Few colorimeters also have aperture or mechanical casing for shielding external light and protecting the internal optics; sometimes voltage regulator attached for stabilizing current supply.
Colorimeter Operating Procedure
- The instrument is switched on by locating the Power Switch knob and rotating it in a clockwise direction (toward the right) and then allowed to warm-up for about 10-15 minutes so that light source and detector are stabilized.
- After warm-up time the appropriate wavelength or filter is selected by turning the Wavelength Control knob (or selecting via mode) for the coloured solution being tested.
- A blank solution (solvent or reagent mixture without analyte) is placed in a clean cuvette and then that cuvette is inserted into the sample chamber, the lid is closed and the display is set to 0 % Transmittance using the “Zero Control knob” or equivalent.
- After the blank calibration the display mode is switched from Transmittance (%T) to Absorbance (A) mode, and if needed the display is adjusted so that 0.0 Absorbance is shown with the blank in place.
- The sample cuvette (solution whose concentration is unknown) is cleaned (wiped to remove fingerprints or bubbles) and inserted in chamber aligning the guide line properly, then the measurement is taken and the reading of %T or A is recorded.
- If a series of standards are run, a calibration curve is constructed (Absorbance vs Concentration) by measuring standard solutions of known concentration at the same wavelength.
- Using the calibration curve the unknown concentration is calculated by relating its measured absorbance to the standards (or using the equation c=Aεlc = \frac{A}{εl}c=εlA if ε and l are constant) .
- After measurement the cuvette is removed, the chamber is closed and the instrument may be turned off; cleaning of the used cuvettes and logging of results are done as standard lab practice.
Types of Colorimeter
There are present different types of Colorimeter, such as;
- A visual/absorption colorimeter is used for simple comparisons where a coloured solution is compared with a standard by eye, and the result is interpreted visually.
- A photo-electric (or photometric) colorimeter is employed in analytical chemistry for measuring absorbance or % transmittance by use of filters and a detector, it gives quantitative results
- A tristimulus colorimeter is designed for measuring colour in terms of how human eye perceives it (for surfaces, paints, textiles), it uses three filters or channels representing RGB or XYZ values.
- A densitometer type (sometimes classed under colorimeters) is used for measuring density of material or optical density in printing/film industry, and they are specialised instruments.
- A spectrophotometric colourimeter (although strictly a spectrophotometer, the name is sometimes used) is capable of scanning wavelengths and though more advanced, it is grouped by some authors under “types of colorimeter” for broader categorisation.
Calibration Procedure for a Colorimeter
Calibration of colorimeter is a crucial part, its done by these following steps;
- The instrument is switched on and allowed to warm up for about 10-15 minutes so that the light source and detector reach stable condition.
- After warm-up the correct wavelength (or filter setting) is selected that matches the test solution, and in some devices the mode is set to % Transmittance or Absorbance as required.
- A blank (solvent or reagent without analyte) is placed in a clean cuvette and inserted into the cuvette holder, the lid is closed and the zero control is adjusted so that the display shows 0.0 Absorbance (or 100.0 %T) with the blank in place.
- After that the display mode may be switched from % Transmittance to Absorbance, and the reading is checked that the blank still reads 0.0 A (or equivalent) else the calibration is corrected.
- The calibration with standards is carried out by measuring known standard solutions (of known concentration) at the selected wavelength, plotting a calibration curve (Absorbance vs Concentration) and then confirming the instrument’s response is linear.
- If drift is noticed or the instrument gives offset readings then the calibration process is repeated and instrument internal offsets are adjusted (or service is called) to prevail accuracy.
- Finally the results and date of calibration are logged (recorded) and the instrument is ready for sample measurement under the same settings used during calibration.
Applications of Colorimeter
- Clinical laboratories are used to determine concentration of substances (like hemoglobin, glucose, proteins) in blood or urine samples by using a colourimeter, which helps in diagnosis of diseases.
- In environmental testing the instrument is applied for analysing water quality (for example levels of iron, chlorine, fluoride or other chemical contaminants) and thereby monitoring pollution or purity of water.
- Food and beverage industry uses colourimeters to ensure consistency of product colour, to check additive concentrations, and to maintain brand standards; the colour and appearance of items are tested for quality control.
- In textile / paint / coatings / printing industries the device is used for matching colours, checking uniformity and ensuring that production batches conform to specified colour standards (and thus reducing waste).
- In microbiology / biochemical research the colourimeter is used for monitoring growth of bacterial cultures, reaction rates of colour-forming assays, by tracking absorbance changes or colour intensity over time.
- In display technology and electronics the colourimeter is used for calibration of monitors/screens (matching RGB values, verifying consistency of visual output) so that what the user sees is accurate compared with standard reference.
Advantages of Colorimeter
- The instrument is offered at a lower cost when compared with more advanced instruments like spectrophotometers, making it economical and accessible for many labs
- Results are produced rapidly which allows real-time monitoring on production lines, and that helps in quality-control without much delay.
- A portable and compact design is provided in many models so that field measurements or on-site inspections can be done easily, they give convenience.
- The operation is simple and intuitive and minimal training is required, thus less skilled personnel can handle it and routine tasks are simplified.
- It allows quantitative analysis of coloured solutions (via absorbance/transmittance) and helps estimation of concentration with reasonable accuracy in many cases.
- Only a small space is required for setup and fewer moving parts are involved, so maintenance and downtime are reduced, making the system sturdy and hardy in many settings.
Limitations of Colorimeter
- The measurement capability is limited because a colorimeter uses only broad-band filters (red, green, blue) rather than full spectrum analysis, so subtler spectral differences are not captured.
- Under different lighting conditions or with metamerism the device’s results may vary and therefore accuracy is compromised, thus it cannot reliably detect colour matches that depend on light source changes.
- Only a certain illuminant and observer angle are built-in (for example D65/2°), so the instrument cannot easily adapt to multiple viewing geometries or lighting conditions in-field.
- For solutions that absorb in UV or infrared ranges the colourimeter is often not suitable, because the device is optimized for visible light (around 400-700nm) and cannot measure outside that effectively.
- The surface texture, gloss, scratches or ambient stray light can influence readings and measurement errors will occur if these factors are not carefully controlled, so extra preparation is required.
- A calibration drift / offset may occur if the instrument is not calibrated regularly, and small changes in light source intensity or detector response will lead to inaccurate results, thus regular service is needed.
- Complex colour effects like fluorescence, pearlescent coatings or metallic finishes are not well handled by a standard colourimeter, they require more advanced spectrophotometric equipment.
Precautions
- The instrument should be placed on a stable, level surface and stray movements are avoided because vibrations or tilt will affect the reading accuracy.
- Before measurement the cuvettes (or sample cells) must be cleaned thoroughly, free of fingerprints, smudges or scratches, since dirty surfaces will distort light path and cause incorrect absorbance.
- It must be ensured that no strong ambient light enters the measurement chamber or passes through sample unexpectedly; stray light will skew results and the instrument’s response will shift slowly.
- The selected wavelength or filter should match the colour of the sample and that decision must be made carefully, wrong filter selection will lead to wrong concentration results.
- The blank (solvent or reagent without analyte) should be measured first and zeroed out before sample runs, failure to do so means baseline drift will occur and errors will prevail
- The cuvette must be placed in correct orientation (markings aligned, path‐length uniform) and bubbles should be removed, else the light beam will be distorted or partly blocked and the reading will be unreliable.
- It should not be operated in humid or dusty environment and the optical ports (windows) must be kept clean and free of powders or liquids, because contamination will degrade performance and shorten lifespan.
- Regular calibration and maintenance must be carried out (lamp hours, detector check, filter drift) since if these are neglected the instrument drift will produce inaccurate data and the credibility will drop.
Colorimeter vs Spectrophotometer – The Difference Between Colorimeter and Spectrophotometer
- The Colorimeter is designed for simple colour measurement by using three primary filters (red, green, blue) and it gives tristimulus values whereas the Spectrophotometer measures the full spectrum of light (wavelength by wavelength) and hence much more detailed spectral data.
- In many routine production / quality-control settings the Colorimeter is preferred because it’s cheaper, easier to use and portable; the Spectrophotometer, on the other hand, is more expensive, more complex and best suited for research or formulation work.
- The Colorimeter is limited in detecting metamerism (the effect where two colours look same under one light but not another) whereas the Spectrophotometer can detect metamerism and handle different illuminants/observer angles.
- The data output from a Colorimeter is generally simpler (for example “% Transmittance” or “Absorbance” or tristimulus XYZ values) while the Spectrophotometer produces spectral curves, full reflectance/transmittance readings and complex results, which enable fine tuning of colour.
- For applications where speed, portability and simplicity are required the Colorimeter is a fit solution, but when extremely high accuracy, multiple wavelength ranges (UV-Vis-IR) or detailed spectral information is needed the Spectrophotometer should be chosen.
- Some trade-offs must be noted: the Colorimeter may suffer from less accuracy or less flexibility in wavelength choice and doesn’t resolve tiny spectral differences, while the Spectrophotometer’s cost, size and training requirements are higher which may not be justifiable for simple tasks.
| Feature | Colorimeter | Spectrophotometer |
|---|---|---|
| Measurement principle | Uses broad-band filters (RGB/tristimulus) to measure a sample’s colour/intensity. | Uses a monochromator/grating to measure absorbance/transmittance at many wavelengths (full spectrum) |
| Wavelength range / flexibility | Narrower band typically visible region (e.g., ~400-700 nm) | Wider range (visible + UV/IR possibilities), multiple wavelengths/geometry options |
| Accuracy & detail | Moderate accuracy for routine tasks; subtle spectral differences may be missed. | Higher accuracy and detailed data; good for research / formulation / complex samples. |
| Cost & complexity | Lower cost, simpler design, easier operation and portable options. | Higher cost, more complex optics/controls/training; often lab-based. |
| Best suited for | Quick colour checks, production QC, field use, simpler samples. | Detailed spectral analysis, research & development, high precision colour matching, metamerism detection. |
Quiz
Which company manufactures the Pocket Colorimeter II?
a) HunterLab
b) X-Rite
c) Hach
d) Vernier
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Hach [/expand]
The Biochrom WPA CO7000 colorimeter is primarily used by:
a) Environmental scientists
b) Textile manufacturers
c) Physicians and medical technicians
d) Cosmetologists
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Physicians and medical technicians [/expand]
Which colorimeter is known for its application in printing, textiles, and cosmetics?
a) Hach Colorimeter
b) HunterLab Colorimeter
c) X-Rite Colorimeter
d) Vernier Colorimeter
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) HunterLab Colorimeter [/expand]
The Laboratory Colorimeter CLR-S (Bioevopeak) is designed to measure substances’ reflected color in:
a) Liquid form only
b) Gas form only
c) Board, powder, and grain forms
d) Solid form only
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Board, powder, and grain forms [/expand]
Which colorimeter offers a more accurate and user-friendly approach than traditional chemical test kits?
a) Pocket Colorimeter II
b) Water Analysis Colorimeter Checker® HC (HANNA Instruments)
c) Environmental Analysis Colorimeter pHotoFlex® (Xylem Analytics)
d) Laboratory Colorimeter WPA CO7000 (Biochrom)
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) Water Analysis Colorimeter Checker® HC (HANNA Instruments) [/expand]
Which colorimeter combines the CIE standard illuminant D65 with a 10° wide viewing field?
a) X-Rite Colorimeter
b) Vernier Colorimeter
c) Laboratory Colorimeter CLR-S (Bioevopeak)
d) Handheld Colorimeter
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Laboratory Colorimeter CLR-S (Bioevopeak) [/expand]
The Environmental Analysis Colorimeter pHotoFlex® (Xylem Analytics) is equipped with how many programs for standard parameters?
a) 50
b) 100
c) 150
d) 180
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: d) 180 [/expand]
Which colorimeter is designed specifically for field and tropical environments?
a) X-Rite Colorimeter
b) Laboratory Colorimeter WPA CO7000 (Biochrom)
c) Handheld Colorimeter
d) Vernier Colorimeter
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) Laboratory Colorimeter WPA CO7000 (Biochrom) [/expand]
Which company’s colorimeter is known for its versatility in measuring a wide array of chemicals?
a) Hach
b) HunterLab
c) X-Rite
d) Vernier
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: a) Hach [/expand]
A handheld colorimeter is primarily designed for:
a) Complex laboratory experiments
b) Field or on-site testing
c) Indoor stationary measurements
d) Underwater testing
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) Field or on-site testing [/expand]
FAQ
How does a colorimeter work?
A colorimeter is an instrument for measuring the colours of light that are reflected or transmitted by a sample. It operates by comparing the sample’s colour to a reference colour and determining the concentration of a given drug based on the sample’s colour intensity.
To use a colorimeter, the user adds a reagent that reacts with the material being measured to the sample. The reaction yields a colourful solution, and the colorimeter measures the color’s intensity. Based on the colour intensity and the known properties of the reagent, the concentration of the substance in the sample can then be determined.
Colorimeters are typically designed to measure a certain range of wavelengths, and they may include wavelength-selecting filters. The sample is placed in a cuvette or other transparent container and illuminated by the colorimeter’s light source. Light is transmitted through the sample before being detected by a detector that monitors the light’s intensity at specified wavelengths. The colorimeter then estimates the concentration of the chemical in the sample by comparing the intensity of the light to a reference colour.
Numerous industries, including water treatment, agriculture, and environmental testing, as well as research and quality control, make extensive use of colorimeters. They are frequently used as an alternative to more complex and costly devices such as spectrophotometers due to their simplicity and precision.
What is another name for a colorimeter?
A colorimeter is also known as a chromometer or a chroma meter. It is a device that measures the colors of light reflected or transmitted by a sample and calculates the concentration of a specific substance in the sample based on the intensity of the color. Colorimeters are widely used in a variety of industries, including printing, textiles, and cosmetics, to ensure consistent color quality. They are also used in research and quality control in industries such as pharmaceuticals, food and beverages, and environmental testing.
What does a colorimeter measure?
A colorimeter measures the light colours reflected or transmitted by an object. It is used to determine the concentration of a certain drug in a sample by comparing the sample’s colour to a standard colour. Typically, the chemical to be measured is added to the sample as a reagent, which interacts with the substance to form a coloured solution. The colorimeter then detects the color’s intensity and determines the sample’s concentration based on the color’s intensity and the known parameters of the reagent.
Colorimeters are utilised in numerous industries, such as printing, textiles, and cosmetics, to assure colour consistency. In industries such as the pharmaceutical, food and beverage, and environmental testing, they are also utilised for research and quality control. Colorimeters are renowned for their user-friendliness and precision, and they are frequently employed in place of more complex and costly devices such as spectrophotometers.
How to use colorimeter?
To use a colorimeter, follow these steps:
Gather all the necessary materials, including the sample, reagent, colorimeter, and cuvette or other transparent container.
Follow the instructions provided with the colorimeter to calibrate the instrument.
Add the appropriate amount of reagent to the sample according to the instructions provided. The reagent will react with the substance to be measured, producing a colored solution.
Place the sample in the cuvette or other transparent container and place it in the colorimeter.
Follow the instructions provided with the colorimeter to activate the instrument and take a reading.
The colorimeter will measure the intensity of the color of the sample and calculate the concentration of the substance based on the intensity of the color and the known properties of the reagent.
Record the results of the test and dispose of the sample and reagent according to local regulations.
It is important to follow the instructions provided with the colorimeter carefully to ensure accurate results. Be sure to wear appropriate protective gear, such as gloves and goggles, when handling chemicals.
How often do you need to calibrate the colorimeter?
The frequency of calibration for a colorimeter depends on a number of factors, including the type of colorimeter, the sensitivity of the instrument, the accuracy required, and the stability of the reference standards used. In general, colorimeters should be calibrated at least once a day or before each use to ensure accurate results. However, more frequent calibration may be necessary in some cases, such as when using a highly sensitive instrument or when working with samples that are prone to changes in pH or temperature.
It is also a good idea to calibrate the colorimeter after any maintenance or repairs, and if it is dropped or subjected to shock or vibration. Consult the manufacturer’s instructions for specific recommendations on the frequency of calibration for your particular colorimeter.
To calibrate a colorimeter, you will need to use reference standards of known concentration. These standards should be as close as possible to the sample being tested in terms of pH, temperature, and other properties that may affect the measurement. Follow the instructions provided with the colorimeter to calibrate the instrument using the reference standards.
What is the principle of colorimeter?
The principle of a colorimeter is based on the absorption of light by a substance. When light passes through a colored solution, some of the light is absorbed by the substance, while the rest is transmitted or reflected. The intensity of the absorbed light is related to the concentration of the substance in the solution.
A colorimeter measures the intensity of the absorbed light at specific wavelengths and compares it to a reference color to determine the concentration of the substance in the sample. To use a colorimeter, the substance to be measured is typically added to the sample in the form of a reagent, which reacts with the substance to produce a colored solution. The colorimeter then measures the intensity of the color and calculates the concentration of the substance in the sample based on the intensity of the color and the known properties of the reagent.
Colorimeters are widely used in a variety of industries, including printing, textiles, and cosmetics, to ensure consistent color quality. They are also used in research and quality control in industries such as pharmaceuticals, food and beverages, and environmental testing. Colorimeters are known for their ease of use and accuracy, and they are often used as an alternative to more complex and expensive instruments such as spectrophotometers.
What is difference between spectrophotometer and colorimeter?
A spectrophotometer is an equipment that measures the amount of light absorbed at specific wavelengths by a substance. By measuring the absorption of light as it passes through a sample, it is used to examine the composition of a substance. Spectrophotometers are frequently used in pharmaceutical, food, and environmental testing industries for scientific research, chemical analysis, and quality control.
A colorimeter is an instrument for measuring the colours of light that are reflected or transmitted by a sample. It measures the colour intensity of a solution to determine the concentration of a drug in a solution. In industries such as printing, textiles, and cosmetics, colorimeters are routinely used to maintain colour consistency.
There are a number of significant distinctions between spectrophotometers and colorimeters:
Sensitivity: Spectrophotometers are more sensitive than colorimeters, meaning they can detect tiny variations in a substance’s concentration.
Accuracy: Spectrophotometers are more precise than colorimeters, with an average measurement error of less than 1%.
Wavelength range: Spectrophotometers can measure a broader spectrum of wavelengths than colorimeters, which are normally built to measure a narrower spectrum.
Cost: Generally speaking, spectrophotometers are more expensive than colorimeters.
Ease of use: Colorimeters are easier to use and more appropriate for applications that do not require a high level of precision. Spectrophotometers are more sophisticated and require more training to operate.
What wavelength does a colorimeter use?
A colorimeter is a device that measures the colors of light reflected or transmitted by a sample. It typically measures a specific range of wavelengths, depending on the substance being measured and the properties of the reagent used.
Colorimeters are designed to measure specific wavelengths of light that are absorbed or reflected by the substance being measured. The wavelengths used may depend on the type of colorimeter, the substance being measured, and the reagent used. For example, some colorimeters may be designed to measure wavelengths in the visible spectrum (400-700 nm), while others may be designed to measure wavelengths in the ultraviolet (UV) or infrared (IR) ranges.
Colorimeters may have filters to select specific wavelengths of light, or they may use a monochromator to separate the light into different wavelengths. The intensity of the absorbed or reflected light is measured at the specific wavelengths of interest, and the concentration of the substance in the sample is calculated based on the intensity of the light and the known properties of the reagent.
What is colorimetry absorbance?
Colorimetry absorbance is a technique for determining the concentration of a material in a sample by measuring the amount of light absorbed by the sample at particular wavelengths. It is founded on the idea that the absorption of light by a material is proportional to the sample’s concentration.
A spectrophotometer or colorimeter is used to beam light through a sample and measure the intensity of the light before and after it passes through the sample. The absorbance of the sample is determined by comparing the light’s intensity before and after passing through the sample. The concentration of the chemical in the sample can then be determined using the absorbance and the material’s known qualities.
Absorbance in colorimetry is widely utilised in scientific research, chemical analysis, and quality control in industries such as pharmaceuticals, food, and environmental testing. It is a fast and accurate method for determining the concentration of a drug in a sample, and it is frequently employed in lieu of more complex and costly analytical procedures.
What is colorimeter used for?
A colorimeter is a device that measures the colors of light reflected or transmitted by a sample and calculates the concentration of a specific substance in the sample based on the intensity of the color. Colorimeters are used for a wide range of applications, including:
Ensuring consistent color quality in industries such as printing, textiles, and cosmetics.
Monitoring the quality of water, air, and soil in environmental testing.
Measuring the nutrient content of soil and water in agriculture.
Ensuring consistent quality and safety in industries such as pharmaceuticals, food and beverages, and cosmetics.
Research and quality control in a variety of industries.
Colorimeters are known for their ease of use and accuracy, and they are often used as an alternative to more complex and expensive instruments such as spectrophotometers. They are widely used in a variety of industries and applications to measure the concentration of specific substances in a sample.
What is the unit of colorimeter?
The unit of measurement for a colorimeter depends on the substance being measured and the reagent used. Colorimeters are used to measure the concentration of specific substances in a sample, and the concentration is typically expressed in units such as milligrams per liter (mg/L), parts per million (ppm), or percent (%) depending on the substance and the scale used.
For example, if a colorimeter is used to measure the concentration of chlorine in a water sample, the concentration may be expressed in units of mg/L or ppm. If a colorimeter is used to measure the pH of a solution, the pH may be expressed on a scale from 0 to 14, with 7 being neutral.
It is important to note that the unit of measurement for a colorimeter may vary depending on the substance being measured and the reagent used. Be sure to consult the instructions provided with the colorimeter to determine the appropriate unit of measurement for your specific application.
What kind of light is used in a colorimeter?
Colorimeters typically use visible light to measure the colors of a sample. Visible light is the portion of the electromagnetic spectrum that is visible to the human eye, and it has wavelengths ranging from about 400 nm to 700 nm.
Colorimeters use filters or a monochromator to select specific wavelengths of light that are absorbed or reflected by the substance being measured. The intensity of the absorbed or reflected light is measured at the specific wavelengths of interest, and the concentration of the substance in the sample is calculated based on the intensity of the light and the known properties of the reagent.
Some colorimeters may also be able to measure wavelengths in the ultraviolet (UV) or infrared (IR) ranges. UV light has shorter wavelengths than visible light and is not visible to the human eye, while IR light has longer wavelengths than visible light and is also not visible to the human eye. Colorimeters that can measure UV or IR wavelengths may be used to measure substances that absorb light at these wavelengths.
What colour filter is used in colorimeter?
A color filter is a transparent or semi-transparent material that absorbs light at certain wavelengths and transmits light at others. Color filters are used in colorimeters to select specific wavelengths of light that are absorbed or reflected by the substance being measured.
The color of the filter is determined by the wavelengths of light that it absorbs. For example, a red filter absorbs light at wavelengths other than red and transmits red light. A blue filter absorbs light at wavelengths other than blue and transmits blue light.
Color filters are used in colorimeters to select specific wavelengths of light that are absorbed or reflected by the substance being measured. The intensity of the absorbed or reflected light is measured at the specific wavelengths of interest, and the concentration of the substance in the sample is calculated based on the intensity of the light and the known properties of the reagent.
The specific color filter used in a colorimeter depends on the substance being measured and the properties of the reagent used. Consult the instructions provided with the colorimeter for more information on the appropriate color filter for your specific application.
Which source is used in colorimeter?
A colorimeter is a device that measures the colors of light reflected or transmitted by a sample. It typically uses a light source to illuminate the sample and a detector to measure the intensity of the light absorbed or reflected by the sample.
The specific light source used in a colorimeter depends on the substance being measured and the properties of the reagent used. Some colorimeters use a tungsten or halogen lamp as the light source, while others use a LED (light-emitting diode) or a xenon lamp. The light source may also be a broadband source that emits light over a wide range of wavelengths, or it may be a monochromatic source that emits light at a specific wavelength.
The light source is typically positioned on one side of the sample and the detector is positioned on the other side. The sample is placed in a transparent container, such as a cuvette, and the light passes through the sample and is absorbed or reflected by the substance being measured. The intensity of the absorbed or reflected light is measured at the specific wavelengths of interest, and the concentration of the substance in the sample is calculated based on the intensity of the light and the known properties of the reagent.
Why 540 nm is used in colorimeter?
The wavelength of 540 nm (nanometers) is often used in colorimeters because it is a wavelength in the green part of the visible spectrum that is absorbed by many substances. The absorption of light at this wavelength is often used to measure the concentration of a specific substance in a sample.
To use a colorimeter, a reagent is typically added to the sample that reacts with the substance to be measured, producing a colored solution. The colorimeter measures the intensity of the color of the solution at specific wavelengths, and the concentration of the substance in the sample can be calculated based on the intensity of the color and the known properties of the reagent.
The specific wavelengths used in a colorimeter may vary depending on the substance being measured and the properties of the reagent used. However, the wavelength of 540 nm is often used because it is absorbed by many substances and is therefore a useful wavelength for a wide range of applications. Consult the instructions provided with the colorimeter for more information on the appropriate wavelengths for your specific application.
What are the parts of colorimeter?
A colorimeter typically consists of the following parts:
Light source: A light source is used to illuminate the sample. The specific light source used in a colorimeter may vary, but it is typically a tungsten or halogen lamp, a LED, a xenon lamp, or a broadband or monochromatic light source.
Sample holder: A sample holder, such as a cuvette, is used to hold the sample in place for measurement. The sample holder is typically made of transparent material, such as glass or plastic, to allow the light to pass through the sample.
Detector: A detector is used to measure the intensity of the light absorbed or reflected by the sample. The detector may be a photodiode, a photomultiplier tube, or other type of light-sensitive device.
Color filter: A color filter is used to select specific wavelengths of light that are absorbed or reflected by the substance being measured. The specific color filter used may vary depending on the substance being measured and the properties of the reagent used.
Display: A display is used to display the results of the measurement. The display may be an LCD screen, a digital readout, or other type of display.
Control panel: A control panel is used to operate the colorimeter and input the necessary parameters for the measurement. The control panel may include buttons, switches, and other controls.
Power source: A power source, such as a battery or AC adapter, is used to power the colorimeter.
Reagent container: A reagent container is used to hold the reagent that
Why use a red filter in a colorimeter?
A red filter is a color filter that absorbs light at wavelengths other than red and transmits red light. It is often used in colorimeters to measure the concentration of a specific substance in a sample by measuring the intensity of the absorbed or reflected red light.
The specific wavelength of red light used in a colorimeter may vary depending on the substance being measured and the properties of the reagent used. Some substances absorb or reflect light more strongly at certain wavelengths of red light, and the colorimeter is calibrated to measure the intensity of the light at these wavelengths.
Using a red filter in a colorimeter can be useful because red light is absorbed by many substances and is therefore a useful wavelength for a wide range of applications. However, the specific color filter used in a colorimeter may depend on the substance being measured and the properties of the reagent used. Consult the instructions provided with the colorimeter for more information on the appropriate color filter for your specific application.
Why is a colorimeter more accurate?
A colorimeter is a device that measures the colors of light reflected or transmitted by a sample and calculates the concentration of a specific substance in the sample based on the intensity of the color. Colorimeters are known for their ease of use and accuracy, and they are often used as an alternative to more complex and expensive instruments such as spectrophotometers.
There are several factors that contribute to the accuracy of a colorimeter:
Wavelength selection: Colorimeters are typically designed to measure specific wavelengths of light that are absorbed or reflected by the substance being measured. By measuring the intensity of the light at these specific wavelengths, the colorimeter can accurately determine the concentration of the substance in the sample.
Calibration: Colorimeters are calibrated using known standards to ensure accuracy. The calibration process involves measuring the intensity of the light at specific wavelengths using a known concentration of the substance being measured and adjusting the colorimeter’s sensitivity to match the known concentration.
Reagent selection: The reagent used in a colorimeter reacts with the substance being measured to produce a colored solution. The specific reagent used can affect the accuracy of the measurement. Selecting a reagent that is specifically designed for the substance being measured can help ensure accurate results.
Sample preparation: Proper sample preparation is important for ensuring accurate results with a colorimeter. The sample should be prepared according to the manufacturer’s instructions and the reagent should be added in the correct proportions.
By carefully selecting the appropriate wavelength, calibrating the colorimeter, selecting the appropriate reagent, and properly preparing the sample, it is possible to achieve accurate results with a colorimeter.
Who discovered colorimeter?
It is difficult to determine who first discovered the principle of colorimetry, as it has likely been used by humans for centuries to measure the colors of substances. However, the modern colorimeter as we know it today was developed in the early 20th century.
The first known patent for a colorimeter was granted in 1908 to a scientist named Georges Demeny. Demeny’s colorimeter used a light source and a photoelectric cell to measure the absorbance of light by a sample at a specific wavelength. The patent described a device that could be used to measure the concentration of a substance in a sample by comparing the absorbance of the sample to a standard solution.
Since Demeny’s early work, the concept of colorimetry has been developed and refined by scientists and engineers, leading to the development of modern colorimeters that are widely used in scientific research, chemical analysis, and quality control in various industries.
Who is the father of calorimetry?
Calorimetry is the science of measuring the heat of chemical reactions and physical changes. While it is difficult to determine who first discovered the principle of calorimetry, as it has likely been used by humans for centuries to measure the heat of substances, several scientists have made significant contributions to the development of calorimetry as a scientific discipline.
One of the earliest known scientists to study calorimetry was Antoine Lavoisier, a French chemist who is considered the “father of modern chemistry.” Lavoisier is credited with establishing the principle of the conservation of mass and developing the concept of heat as a form of energy. He conducted experiments to measure the heat of chemical reactions and physical changes, and he developed a calorimeter to measure the heat of combustion.
Other notable scientists who have made significant contributions to the development of calorimetry include James Joule, who is known for his work on the concept of the mechanical equivalent of heat, and Sadi Carnot, who developed the concept of the Carnot cycle, which is the basis for the study of thermodynamics.
- Product manuals: Omega engineering (no date) Product Manuals | Omega Engineering. LaMotte. Available at: https://www.omega.com/en-us/pdf-manuals.
- A guide to colorimetry – cole-parmer (no date). Sherwood Scientific . Available at: https://archive-resources.coleparmer.com/Manual_pdfs/Sherwood/ChromaColorimeters/Manuals/AGuidetoColorimetry.pdf.
- Colorimeter User Manual – Vernier (no date). Available at: https://www.vernier.com/manuals/col-bta/
- https://www.sciencedirect.com/topics/engineering/colorimeter
- https://www.vedantu.com/chemistry/colorimeter
- https://laboratorytests.org/colorimeter/
- https://collegedunia.com/exams/colorimeter-chemistry-articleid-681
- https://paramedicsworld.com/biochemistry-practicals/demonstration-of-colorimeter-principle-components-working-uses-applications/medical-paramedical-studynotes
- https://www.toppr.com/guides/chemistry/solutions/colorimeter-definition-and-uses-of-colorimeter/
- https://www.testronixinstruments.com/blog/working-principle-applications-of-colorimeters/
- https://www.hunterlab.com/blog/lab-vs-lch-coordinates/
- https://microbeonline.com/colorimeter-principles-parts-types-and-uses/
- https://www.azosensors.com/article.aspx?ArticleID=324
- https://infinitylearn.com/surge/chemistry/colorimeter/
- https://www.hunterlab.com/blog/lab-vs-lch-coordinates/
- https://www.amu.ac.in/department/bio-chemistry-jnmc/sops
- https://www.tumblr.com/microamaze/134454969515/precaution-when-using-colorimeter
- http://www.xzbelec.com/news/industry-news/precautions-on-using-of-colorimeter.html
- https://www.ecstuff4u.com/2021/05/advantages-disadvantages-colorimeter.html?m=1
- https://microbenotes.com/colorimeter-definition-principle-parts-uses-examples/