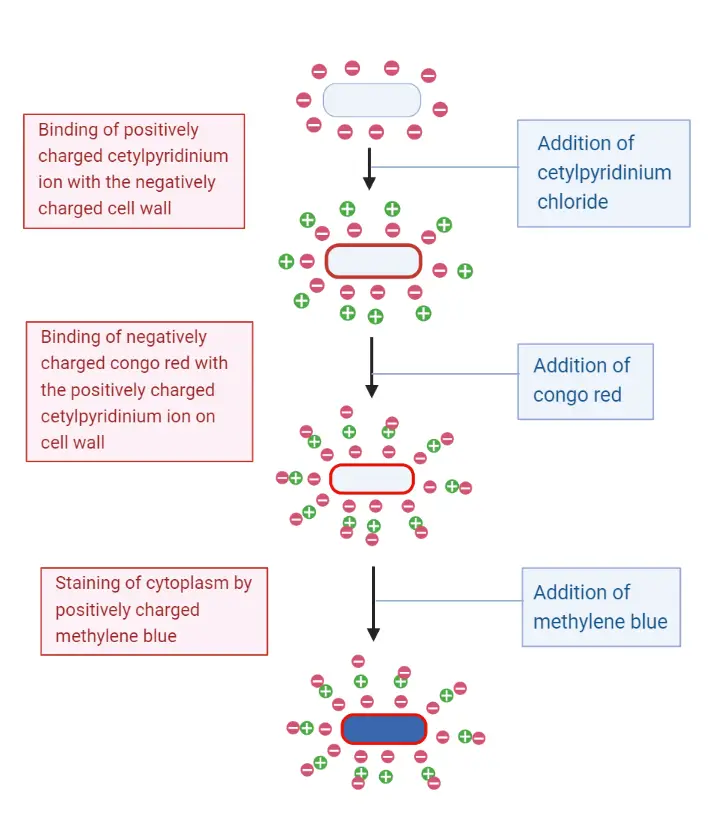
Cell wall staining by Dyar’s method is an indirect staining process that is used to visualize the bacterial cell wall boundary. It is the method introduced by M. T. Dyar in 1947 and it is based on the surface charge present on the bacterial cell. It is the cell wall that normally carries a net negative charge, and this is used for forming an adsorbed complex during staining.
In this method the smear is first treated with Cetylpyridinium chloride (CPC) which is a cationic surface-active agent. It is adsorbed on the cell wall and it is the step that reverses the surface charge into a positive one. This modified surface now attracts Congo red which is an acidic dye (anionic). The dye binds firmly with the CPC layer and a red coloured precipitated complex is formed on the cell wall. It is the precipitated layer that makes the cell wall boundary clear. In earlier procedures osmium tetroxide (OsO₄) fixation was used before staining, because it helps in stabilizing this delicate adsorbed complex.
After Congo red treatment a basic counterstain such as methylene blue is added. This dye enters into the cell and stains the cytoplasm blue. It is repelled by the positively charged cell wall complex, so the boundary remains red. Hence the stained cell shows a red coloured cell wall around a blue coloured cytoplasm, giving a clear differentiation between the wall and internal contents.
Objective of Cell Wall Staining
The main purpose of cell wall staining is to identify the cell wall containing bacterial cells and study the detailed morphology of the cell wall.
Principle of Cell Wall Staining by Dayr’s Method
The principle of Dyar’s method is based on the charge present on the bacterial cell surface. It is known that the bacterial cell wall carries a net negative charge and this charge is used for forming an adsorbed staining complex. In this method the smear is first treated with Cetylpyridinium chloride (CPC) which is a cationic reagent. The positively charged CPC ions are adsorbed on the negatively charged wall and it changes the surface into a temporary positive one. This is the step that prepares the wall for receiving an acidic dye.
After the CPC treatment the smear is flooded with Congo red. Congo red is an acidic dye (anionic) and it is strongly attracted towards the CPC coated wall. The dye forms a precipitated complex on the wall and this gives the red colour to the cell boundary. It is this colour formation that marks the position of the cell wall.
A basic counterstain such as methylene blue is finally applied. The basic dye is repelled by the positive red-stained wall but it enters the cell and stains the cytoplasm blue. It is the charge-based adsorption of dyes that helps in marking the wall distinctly as a red outline around a blue interior.
Requirement
I. Biological Material
- A bacterial culture is required.
- A young or 24 hours old culture is generally used for staining.
II. Chemical Reagents (Stains and Differential Agents)
- Cetylpyridinium chloride (CPC) solution is needed. It is the cationic reagent used as mordant and also for reversing the surface charge.
- Congo red solution is required. It is the acidic dye used for forming the red coloured complex on the wall.
- Methylene blue is used as the basic counterstain for staining the cytoplasm.
- Distilled water is used in rinsing steps during the staining.
III. Chemical Reagent (Fixation – Classical Method)
- Osmium tetroxide (OsO₄) is required for fixation. It is used for stabilizing the envelope and gives better results in the classical procedure.
IV. Equipment and Materials
- Clean glass slide for smear preparation.
- Inoculating loop for transferring the culture.
- Dropper for adding reagents.
- Bunsen burner used in sterilizing the loop, air drying and heat fixing.
- Light microscope is needed for observation, and oil immersion lens is normally used.
Procedure of Cell Wall Staining
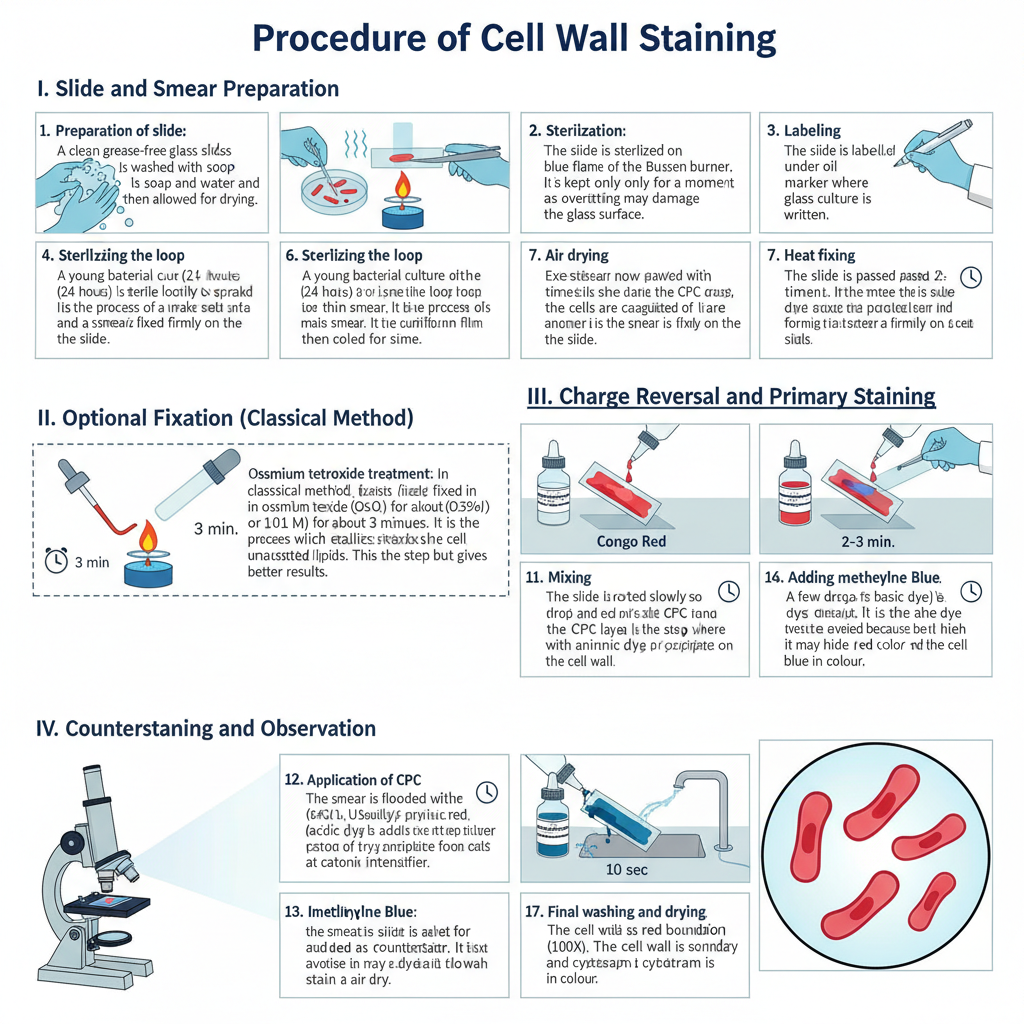
I. Slide and Smear Preparation
1. Preparation of slide
It is done by taking a clean grease-free glass slide. The slide is washed with soap and water and then allowed for drying.
2. Sterilization
The slide is sterilized on the blue flame of the Bunsen burner. It is kept only for a moment as overheating may damage the glass surface.
3. Labelling
The slide is labelled with a glass marker where the name of the culture is written.
4. Sterilizing the loop
The wire loop is heated on the flame till it becomes red hot. It is then cooled for some time.
5. Smear preparation
A young bacterial culture (24 hours) is taken with the sterile loop and spread gently to make a thin smear. It is the process of making a uniform film on the slide.
6. Air drying
The smear is now allowed to air dry completely.
7. Heat fixing
The slide is passed 2–3 times over the flame. In this step, proteins of the cells is coagulated and the smear is fixed firmly on the slide.
II. Optional Fixation (Classical Method)
1. Osmium tetroxide treatment
In the classical method, the smear is fixed in osmium tetroxide (OsO₄) for about 3 minutes. It is the process which stabilizes the cell envelope by reacting with unsaturated lipids. This step is optional but gives better results.
III. Charge Reversal and Primary Staining
1. Application of CPC
The smear is flooded with cetylpyridinium chloride (CPC). Usually 3–4 drops of CPC solution (0.35% or 0.01 M) is used. It acts as a cationic intensifier.
2. Adding Congo red
Immediately few drops of Congo red solution (acidic dye) is added over the CPC layer.
3. Mixing
The slide is rotated slowly so that CPC and Congo red mix properly on the smear. It is the step where the cationic layer reacts with the anionic dye forming a red precipitate on the cell wall.
4. Incubation
The slide is kept undisturbed for about 2–3 minutes at room temperature.
5. Washing
Excess stain is washed off with distilled water.
IV. Counterstaining and Observation
1. Adding methylene blue
A few drops of methylene blue (basic dye) is added as counterstain. It is the dye which stains the cytoplasm.
2. Incubation
The smear is kept for about 10 seconds. Overstaining is avoided because it may hide the red color of the cell wall.
3. Final washing and drying
The slide is washed well with distilled water and allowed to air dry.
4. Microscopy
The smear is observed under oil immersion (100X). The cell wall is seen as a red boundary and the cytoplasm is blue in colour.
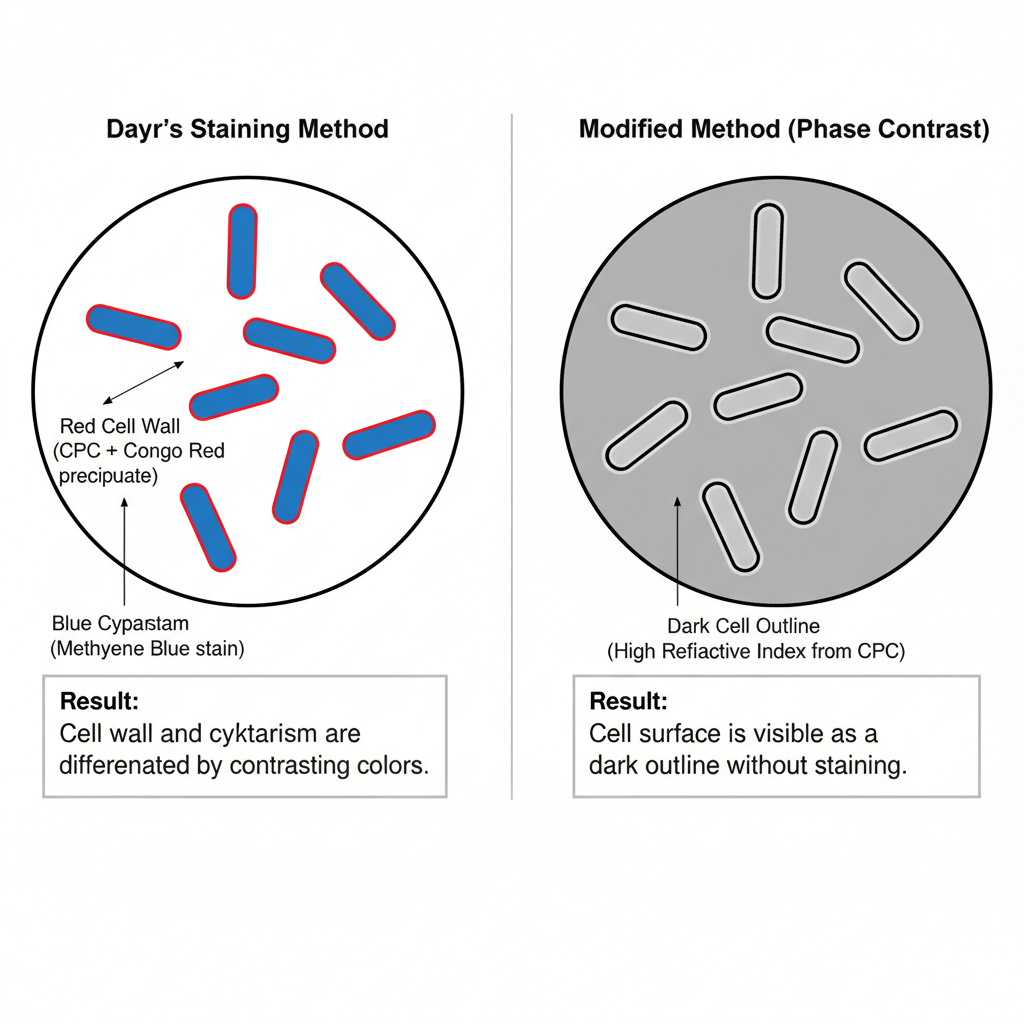
Result and Interpretation
The result of Cell Wall Staining by Dayr’s Method is seen clearly under oil immersion objective. It is the stage where the cell wall and the cytoplasm become differentiated by two contrasting dyes.
The cell wall is seen as a red boundary. This red colour is produced due to the reaction between the cationic CPC and the acidic Congo red which forms a red precipitated layer only on the outer wall. It is localized on the surface and does not enter inside the cell.
The cytoplasm is blue in colour. This is due to methylene blue which enters the cell easily and binds with the inner structures. Because the red stained wall repel the basic dye, the blue stain only appears inside the cell.
The appearance of a red outer wall and a blue cytoplasm indicates the presence and integrity of the cell wall. It also shows the outline of other structures like yeast wall and outer coats of endospores.
Result of Modified (Non-Staining) Method
In the modified method where no dye is used, the cells is only kept in CPC solution. Under phase contrast microscope the cell surface appears as a dark outline. It is the effect of CPC which changes the refractive index of the cell boundary. These are useful for seeing the cell surface and septa quickly without staining.
Limitation of Dayr’s Method
- The method is sensitive to timing during staining and counterstaining steps.
- Overexposure of methylene blue hide the red cell wall colour.
- Improper mixing of CPC and Congo red does not form the required precipitate layer.
- Thick smear preparation makes overlapping of cells which affect observation.
- Excess washing remove the CPC–Congo red complex from the cell wall.
- Incomplete drying may cause the smear to float during microscopy.
- Overheating during fixation distort the cell structure and affect CPC binding.
- Osmium tetroxide (OsO₄) used in the classical method is toxic and hazardous.
- The CPC–Congo red layer is unstable and easily disturbed under different conditions.
- Gram-positive cells already stained with CV–I complex do not show proper cell wall staining.
- Prolonged methylene blue exposure obscure the red wall stain.
- It is not commonly used now because of sensitivity and safety issues.
- High magnification and bright field observation is required for proper visualization.
Advantages of Dayr’s Method
- It gives a clear red boundary of the cell wall with a blue cytoplasm, helping to identify the cell wall position.
- It is useful for studying cell wall morphology under bright field microscope.
- The method shows charge reversal on the cell surface by using CPC, which help in binding of Congo red.
- It can also show the outer coats of endospores and yeast cell walls.
- It is helpful in teaching because it demonstrates the exact location of the cell wall.
- It was important historically in studying cell wall structure and understanding Gram reaction.
- The modified method with only CPC is fast and does not need dyes or OsO₄.
- The rapid CPC method show clear surface outline under phase contrast microscope.
- The modification is suitable for both Gram-positive and Gram-negative cells.
- It can also show cell septa along with the cell surface.
Uses of Dayr’s Method
- It is used to see the cell wall boundary clearly by giving a red outline around the cell.
- It helps in studying the morphology and structure of the cell wall.
- The method can show the outer coats of endospores and yeast cell walls.
- It was used in early research to understand the location of cell wall in relation to Gram reaction.
- It helped in explaining how the CV–I complex in Gram-positive cells interfere with Dyar staining.
- It provided a structural reference point for testing different hypotheses on Gram differentiation.
- The method demonstrates the principle of charge reversal on bacterial surface by using CPC.
- It is useful in teaching because it shows the physical position of the cell wall very clearly.
- The modified CPC method is used for quick surface observation under phase contrast microscope.
- The rapid modification works for both Gram-positive and Gram-negative bacteria.
- It can also be used to show cell septa along with the cell surface.
- It is helpful in cytological studies where surface charge and wall integrity are examined.
- Ahern, H. (n.d.). Differential Staining Techniques. In Microbiology: A Laboratory Experience. Milne Publishing.
- Belazi, D., Sole Domenech, S., Johansson, B., Schalling, M., & Sjövall, P. (2009). Chemical analysis of osmium tetroxide staining in adipose tissue using imaging ToF-SIMS. Histochemistry and Cell Biology, 132(1). https://doi.org/10.1007/s00418-009-0587-z
- CellaVision. (2022). What are the main problems you encounter when Gram staining?
Dyar, M. T. (1947). A Cell Wall Stain Employing a Cationic Surface-active Agent as a Mordant. - Journal of Bacteriology, 53(4), 498. https://doi.org/10.1128/jb.53.4.498-498.1947
- Editorial Team. (2024, May 18). Cell Wall Staining by Dayr’s Method. Biology Notes Online.
- Jones, C. J., & Wozniak, D. J. (2017). Congo Red Stain Identifies Matrix Overproduction and Is an Indirect Measurement for c-di-GMP in Many Species of Bacteria. In Methods in Molecular Biology (Vol. 1657, pp. 147–156). https://doi.org/10.1007/978-1-4939-7240-1_12
- MilliporeSigma. (2021). Calcofluor White Stain (Technical Bulletin No. 18909).
- Neville, M. E., & Holt, J. G. (1975). Rapid method for demonstrating cell surfaces of bacteria using phase contrast microscopy. Transactions, Illinois State Academy of Science, 68(3), 288–295.
- Nielson, A. J., & Griffith, W. P. (1978). Tissue fixation and staining with osmium tetroxide: the role of phenolic compounds. Journal of Histochemistry and Cytochemistry, 26(2), 138–140. https://doi.org/10.1177/26.2.75221
- Sumi Home Decor (Microbiology with Sumi). (n.d.). Cell wall Staining by Dyar’s Method [Video]. YouTube.
- Tripathi, N., Zubair, M., & Sapra, A. (2025). Gram Staining. In StatPearls [Internet]. StatPearls Publishing.
- [Untitled Expert Report: The Dyar Cell Wall Staining Method—Mechanistic Analysis, Historical Context, and Modern Cytological Relevance]. (n.d.).
- Wikipedia contributors. (n.d.). Calcofluor-white. In Wikipedia. Retrieved.
- Wikipedia contributors. (n.d.). Staining. In Wikipedia. Retrieved.
- Yakupova, E. I., Bobyleva, L. G., Vikhlyantsev, I. M., & Bobylev, A. G. (2019). Congo Red and amyloids: history and relationship. Biochemical Society Transactions, 39(1):BSR20181415. https://doi.org/10.1042/BSR20181415