Carbon cycle Definition
Carbon cycle is the process by which carbon compounds move between the earth’s atmosphere, biosphere, geosphere, pedosphere, and hydrosphere.
- The biogeochemical process by which carbon moves between the Earth’s biosphere, pedosphere, geosphere, hydrosphere, and atmosphere is called the carbon cycle.
- Carbon is the most important part of all living things and a big part of many minerals, like limestone.
- Along with the nitrogen cycle and the water cycle, the carbon cycle is a series of important events that make it possible for life to exist on Earth. It talks about how carbon moves around the biosphere as it is recycled and reused, as well as the long-term processes of carbon being stored in carbon sinks and released from them.
- At the moment, carbon sinks on land and in the ocean each take in about a quarter of the carbon dioxide that people make.
- Humans have been messing with the biological carbon cycle for hundreds of years by changing how land is used and by mining fossil carbon (coal, oil and gas extraction, and making cement) on an industrial scale from the geosphere.
- By 2020, the amount of carbon dioxide in the atmosphere was nearly 52% higher than it was before people started using factories. This meant that the Sun had to heat the atmosphere and Earth’s surface more.
- Due to dissolved carbon dioxide, carbonic acid, and other compounds, the increase in carbon dioxide has also made the surface of the ocean about 30% more acidic. This is changing the chemistry of the ocean in a big way.
- Most of the fossil carbon has been taken out in the last 50 years, and the rate of taking it out keeps going up quickly, which contributes to climate change caused by people.
- Due to the large but limited inertia of the Earth system, the biggest effects on the carbon cycle and the biosphere, which is essential for human civilization, are still to come.
- The Paris Climate Agreement and Sustainable Development Goal 13 both say that restoring balance to this natural system is a top international goal.
Properties of Carbon
Physical Properties of Carbon
- Carbon stands alone as a chemical element. It manifests itself in a wide variety of manifestations. Coal and soot are two materials that contain pure carbon.
- It’s either a muted grey or a flat black, and it’s quite boring and subtle.
- Charcoal, produced by burning carbon in the absence of air, is one of the most useful carbon compounds.
- It manifests in a variety of allotropic manifestations. Various allotropes of a given element exist, each with its own unique set of chemical and physical properties.
- Carbon densities vary depending on where the element was first created. There are pure forms of carbon like diamonds and emeralds, and impure forms like coal, which combines carbon and hydrogen.
Chemical Properties of Carbon
- Carbon compounds generally show 4 reactions, they are
- Combustion reaction
- Oxidation reaction,
- Addition reactions
- Substitution reaction.
- As everyone knows, carbon requires oxygen, heat, and light to make carbon dioxide. When something is burned in the air to produce carbon dioxide, this process is known as combustion.
- Let us illustrate this with a few scenarios when it is burned in the air: When methane is burned in the presence of oxygen, carbon dioxide, heat, and light are produced.
Steps of Carbon Cycle
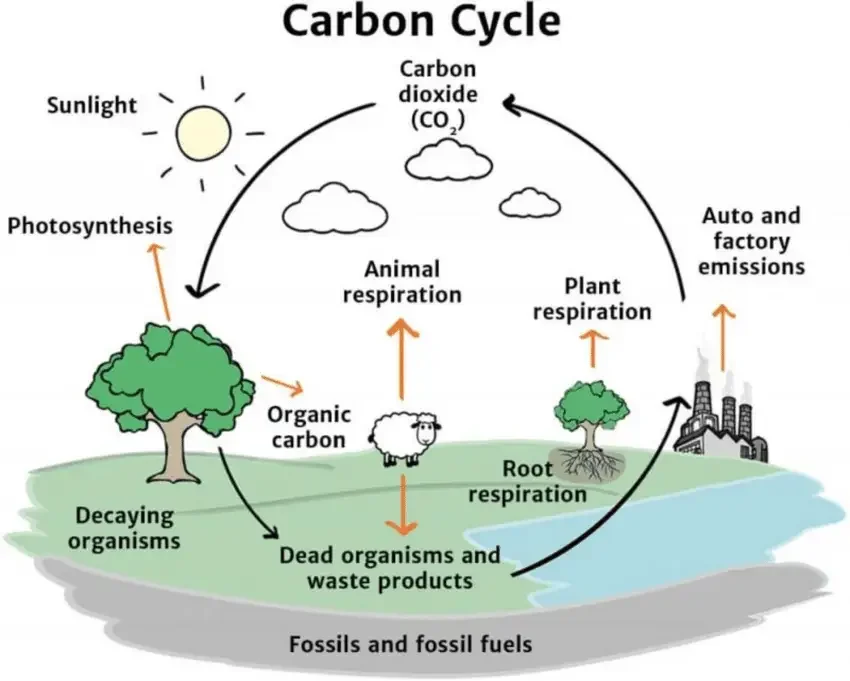
1. Carbon in the Atmosphere
- For carbon atoms to enter the carbon cycle, they must first exist in a gaseous state.
- CO2 can be produced either by inorganic mechanisms or by the metabolic processes of living organisms.
- Carbon dioxide gas likely originated from volcanic activity and asteroid collisions before life existed on Earth.
- Carbon is currently released into the atmosphere through the activity of living things, such as animal exhalations, decomposer organisms, and human combustion of wood and fossil fuels.
- Regardless matter how carbon dioxide enters the atmosphere, CO2 gas is the beginning of the carbon cycle.
2. Producers Absorb Carbon
- Organisms that make food from sunlight, such as plants, absorb carbon dioxide from the atmosphere and use it to construct carbohydrates, lipids, proteins, and other important biomolecules.
- CO2 is absorbed by plants through pores in their leaves known as stomata. Carbon dioxide enters the plant through the stomata and, with the aid of solar energy, is integrated into carbon-containing molecules.
- The ability of plants and other producer organisms such as cyanobacteria to convert atmospheric carbon into living matter is essential to life on Earth.
3. Producers are Eaten
- Consumers are species that consume other organisms. Animals are the most obvious sort of consumer in our ecosystems, however numerous species of microorganisms also belong to this category.
- Incorporating carbon molecules from plants and other dietary sources is a function of ingestion. They use some of these carbon molecules from food to construct their bodies, but the majority of the food they consume is broken down to release energy, which is virtually the opposite of what producers do.
- Animals break these bonds to liberate the energy they contain, converting sugars, lipids, and other carbon molecules into single-carbon units. These are eventually discharged into the atmosphere as carbon dioxide.
4. Decomposers Release Carbon
- Plants and animals that die without being consumed by other organisms are decomposed by other organisms known as “decomposers.”
- Decomposers consist of numerous bacteria and a few fungi. They typically exclusively decompose dead stuff, as opposed to catching and devouring living animals or plants.
- Decomposers, like mammals, degrade the chemical bonds in their food molecules. They produce numerous chemical byproducts, including CO2 in some instances.
5. Human Activities
- Humans have recently made significant modifications to the carbon cycle on Earth. By burning vast quantities of fossil fuels and removing about half of the Earth’s forests, people have reduced the planet’s ability to remove carbon from the atmosphere, while releasing vast quantities of carbon that had been stored in solid form as plant matter and fossil fuels.
- This would increase the amount of carbon dioxide in the Earth’s atmosphere, which is problematic because carbon dioxide is a “greenhouse gas” that regulates the Earth’s temperature and weather patterns.
- The scientific community has expressed concern that by significantly altering the Earth’s carbon cycle, we may end up altering our climate or other crucial components of the ecosystem on which our survival depends.
- As a result, many experts suggest reducing carbon emissions by reducing automobile use and electricity use, and investing in non-combustible energy sources such as solar and wind power.
Summary of Carbon Cycle Steps
The carbon cycle process consists of the following major steps:
- Plants absorb carbon from the atmosphere during photosynthesis.
- These plants are then devoured by animals, resulting in the bioaccumulation of carbon within their bodies.
- These organisms eventually perish, and their decomposition releases carbon back into the atmosphere.
- Some of the carbon that is not returned to the atmosphere becomes fossil fuels over time.
- These fossil fuels are subsequently utilised in human activities, which release additional carbon into the atmosphere.
Syntrophy and Methanogenesis
- Most organic compounds are oxidized in nature by aerobic microbial processes.
- However, because oxygen (O2) is a poorly soluble gas and is actively consumed when available, much organic carbon still ends up in anoxic environments.
- Methanogenesis, the biological production of CH4, is a major process in anoxic habitats and is catalyzed by a large group of Archaea, the methanogens, which are strict anaerobes.
- Most methanogens can use CO2 as a terminal electron acceptor in anaerobic respiration, reducing it to CH4 with H2 as electron donor.
- Only a very few other substrates, chiefly acetate, are directly converted to CH4 by methanogens.
- To convert most organic compounds to CH4, methanogens must team up with partner organisms called syntrophs that function to supply them with precursors for methanogenesis.
What is the Fast carbon cycle?
- There are both rapid and sluggish carbon cycles.
- The fast cycle occurs in the biosphere, whereas the slow cycle occurs in rocks.
- The rapid or biological cycle can be completed within a few years, transferring carbon from the biosphere to the atmosphere and back again.
- The fast carbon cycle involves biogeochemical activities between the environment and living organisms in the biosphere that are particularly short-term (see diagram at start of article).
- It comprises carbon exchanges between the atmosphere, terrestrial and marine ecosystems, soils, and sediments on the seafloor.
- The fast cycle is comprised of annual cycles of photosynthesis and decadal cycles of vegetative growth and breakdown.
- Many of the more immediate effects of climate change will be determined by the rapid carbon cycle’s responses to human activities.
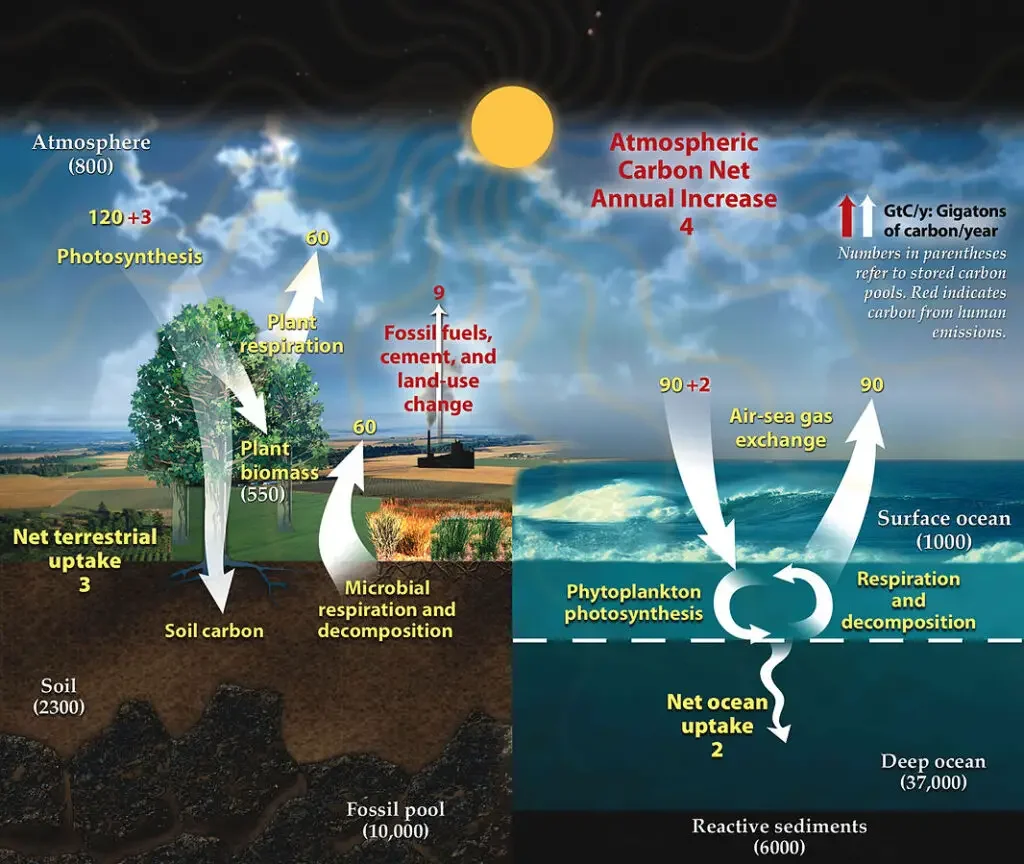
What is the Slow carbon cycle?
- It can take millions of years for the slow or geological cycle to move carbon through the Earth’s crust through rocks, soil, ocean, and atmosphere.
- The slow carbon cycle involves geochemical processes with a medium to long timescale that are part of the rock cycle (see diagram on the right).
- The interchange between the water and atmosphere can take centuries, whereas rock weathering can take millions of years.
- Carbon in the ocean precipitates to the ocean floor, where it can form sedimentary rock and be subducted into the mantle of the earth.
- This geological carbon returns to Earth’s surface as a result of mountain-building processes. There, rocks are eroded and carbon is returned to the atmosphere through degassing and to the ocean via rivers.
- Other geologic carbon returns to the ocean via hydrothermal calcium ion emission.
- In a given year, 10 to 100 million tonnes of carbon circulate through this sluggish cycle. This includes volcanoes releasing geologic carbon as carbon dioxide directly into the atmosphere.
- However, this represents less than one percent of the carbon dioxide produced by the combustion of fossil fuels.
Terrestrial carbon in the water cycle
- As cloud condensation nuclei, atmospheric particles promote cloud formation.
- Raindrops acquire organic and inorganic carbon through particle scavenging and vapour absorption as they fall to Earth.
- Burning and volcanic eruptions generate highly condensed polycyclic aromatic molecules (i.e., black carbon), which are returned to the environment alongside greenhouse gases such as CO2.
- Terrestrial plants fix atmospheric CO2 via photosynthesis and return a portion to the atmosphere via respiration. Lignin and celluloses account for up to 80% of the organic carbon in forests and 60% of the organic carbon in pastures.
- Litterfall and root organic carbon combine with sedimentary material to generate organic soils in which plant-derived and petrogenic organic carbon is both stored and changed by the activities of microorganisms and fungi.
- Water absorbs dissolved organic carbon (DOC) and dissolved inorganic carbon (DIC) as it flows over forest canopies (i.e. throughfall) and along plant trunks/branches (i.e. stemflow). Biogeochemical changes occur as water percolates into soil solution and groundwater reservoirs. Overland flow happens when soils are totally saturated or when precipitation comes faster than soil saturation.
- Organic carbon derived from the terrestrial biosphere and in situ primary production is decomposed by microbial communities in rivers and streams in conjunction with physical decomposition (i.e. photo-oxidation), resulting in a flux of CO2 from rivers to the atmosphere of the same magnitude as the amount of carbon sequestered annually by the terrestrial biosphere.
- Macromolecules produced from the earth, such as lignin and black carbon, are broken into smaller components and monomers, which are ultimately transformed to CO2, metabolic intermediates, or biomass.
- Lakes, reservoirs, and floodplains often store substantial quantities of organic carbon and sediments, but also experience net heterotrophy in the water column, resulting in a net flow of CO2 to the atmosphere that is approximately one order of magnitude lower than rivers.
- In addition to floodplains, lakes, and reservoirs, anoxic sediments of floodplains, lakes, and reservoirs are often rich in methane generation. Typically, the export of fluvial nutrients causes an increase in primary production in river plumes. Nonetheless, estuary waters represent a global source of CO2 for the atmosphere. Blue carbon is both stored and exported by coastal wetlands. Globally, marshes and wetlands are believed to release the same amount of CO2 into the atmosphere as rivers.
- Typically, continental shelves and open oceans absorb CO2 from the atmosphere.
- The marine biological pump stores a tiny but significant portion of the absorbed CO2 in marine sediments as organic carbon (see next section).
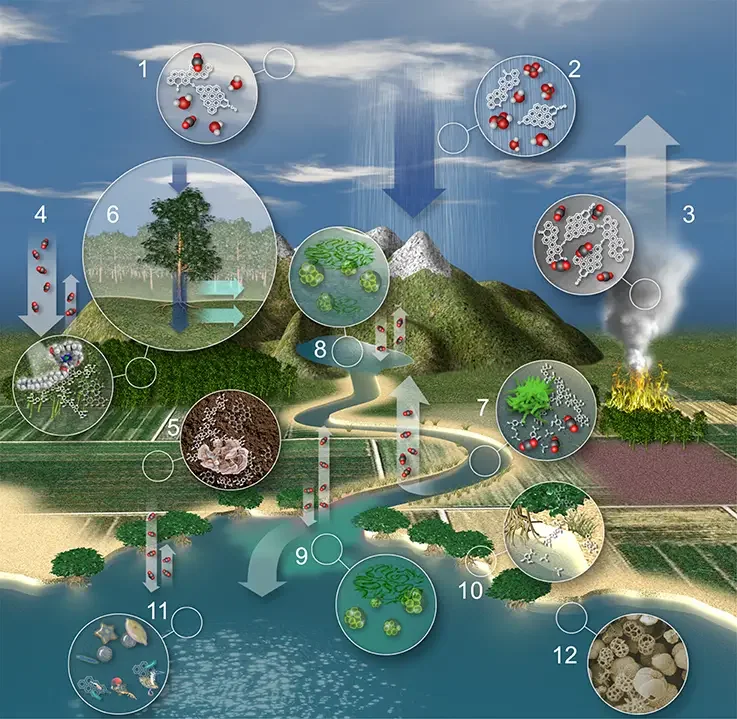
What is the Deep Carbon cycle?
- The deep carbon cycle is the geochemical transport of carbon through the mantle and core of the Earth.
- It is intricately tied to the transport of carbon on the Earth’s surface and in the atmosphere as part of the carbon cycle.
- By returning carbon to the deep Earth, it serves a crucial role in preserving the conditions on Earth required for life. Without it, carbon would build up in the atmosphere, reaching extraordinarily high quantities over extended time periods.
- Due to the inaccessibility of the deep Earth to drilling, little is clearly known about the role of carbon un it.
- Nonetheless, multiple pieces of evidence, the most of which originate from laboratory models of deep Earth conditions, have shown the mechanisms for the element’s journey down into the lower mantle and the shapes that carbon takes at the severe temperatures and pressures of this layer.
- In addition, tools such as seismology have improved our understanding of the possible presence of carbon in the Earth’s core.
- Studies of the composition of basaltic lava and the flux of carbon dioxide from volcanoes indicate that the amount of carbon in the mantle is one thousand times greater than that on the Earth’s surface.
Effects of human activities and environmental phenomena
- Carbon dioxide (CO2), a greenhouse gas, is rapidly released into the atmosphere by the combustion of fossil fuels, which raises average world temperatures and causes ocean acidification.
- Agricultural practises that generate carbon dioxide and methane (CH4, a greenhouse gas) emissions. For instance, methane is produced through the digestion of plant matter by cows and the proliferation of bacteria in rice fields. Carbon dioxide is emitted by the combustion of fossil fuels to power agricultural machinery, the mining of minerals, and the production of fertiliser. Crop production and livestock husbandry also influence local productivity and biomass, as well as the rates of photosynthesis, respiration, and decomposition of organic matter.
- Deforestation reduces photosynthesis rates and consequently the amount of carbon dioxide collected by plant growth. As trees develop, they absorb atmospheric carbon dioxide and store it in their wood, leaves, bark, and roots. Carbon is returned to the atmosphere when downed trees are allowed to rot or when they are deliberately set on fire, a typical method of deforestation. Therefore, deforestation typically results in the emission of carbon dioxide, unless all of the wood is used for construction or paper products.
- The area of permafrost (permanently frozen soil) that contains methane (CH4, a greenhouse gas). When temperatures remain cold throughout the year, organic matter decomposes very slowly and remains in the soil. Methane is released when permafrost melts, which is occurring as global temperatures rise. Increasing temperatures also accelerate the rate of decomposition, which raises the concentration of greenhouse gases in the atmosphere.
- Changes in the rates of sedimentation and burial of organic matter impact the quantity of carbon available for decay and the amount of carbon stored in the rock record over millions of years. Increased burial of dead plants and plankton, for instance, reduces decomposition, hence accelerating the creation of fossil fuels.
- Over millions of years, the rock cycle can alter the concentration of carbon dioxide in the atmosphere. Under heat and pressure, for instance, metamorphic events can release carbon dioxide. In contrast, the weathering of rocks that occurs when carbon dioxide dissolves in rainwater to generate carbonic acid (H2CO3) reduces the atmospheric concentration of carbon dioxide. These weathering reactions can be accelerated by warming, but not at a rate sufficient to offset the increase in carbon dioxide caused by human activities.
- Geologic variations in the pace of volcanism, caused by plate tectonics, can significantly influence the amount of carbon dioxide in the atmosphere, but over millions of years, not human timescales.
Examples of Carbon Cycle
The carbon cycle is composed of numerous parallel processes that can absorb or release carbon. Together, these processes maintain the relative stability of the Earth’s carbon cycle, temperature, and biosphere. Listed below are ecological components that can absorb carbon, convert carbon to living matter, or release carbon back into the atmosphere.
Atmosphere
- Carbon dioxide in the Earth’s atmosphere is an important reservoir of carbon. In conjunction with two oxygen atoms, carbon produces a stable, gaseous molecule.
- This gas is released in nature by volcanic activity and by the breathing of animals, which attach carbon molecules from the food they ingest to oxygen molecules before exhaling it.
- Plants can remove carbon dioxide from the atmosphere by converting atmospheric carbon into sugars, proteins, lipids, and other life-sustaining chemicals.
- Carbon dioxide can also be removed from the atmosphere by absorption in the ocean, where water molecules can combine with carbon dioxide to generate carbonic acid.
Lithosphere
- The Earth’s crust, which is known as the “lithosphere” from the Greek words for “stone” and “globe,” can also leak carbon dioxide into the atmosphere.
- This gas can be produced by chemical reactions in the crust and mantle of the Earth.
- Volcanic activity can result in natural carbon dioxide emissions.
- Some experts suggest that widespread volcanism may have contributed to the global warming that triggered the Permian extinction.
- However, the Earth’s crust may also take carbon from the atmosphere. Movements of the Earth’s crust can bury carbon-containing compounds far underground, where their carbon cannot escape back into the atmosphere.
- Over millions of years, these subsurface organic matter stores transform into coal, oil, and gasoline.
- In recent years, people have began releasing a significant portion of this carbon back into the atmosphere by burning these materials to power automobiles, power plants, and other equipment.
Biosphere
- Some organisms extract carbon dioxide from the atmosphere, while others release it. Plants and animals are the most prominent members of this ecosystem.
- Carbon is removed from the atmosphere by plants. This is not a benevolent gesture; plants actively need atmospheric carbon as “food” to produce sugars, proteins, lipids, and other vital components for life.
- Carbon dioxide and other trace elements are used to create these organic compounds by plants through photosynthesis, which harvests the energy of sunshine. Indeed, “photosynthesis” is derived from the Greek terms “photo” for “light” and “synthesis” for “to combine.”
- In a delicately balanced series of chemical events, animals consume plants (and other animals) and then disassemble these newly created molecules.
- During photosynthesis, plants store chemical energy in the bonds between carbon atoms and other atoms. Animals obtain their energy from these bonds.
- To accomplish this, animal cells break down complex compounds such as carbohydrates, lipids, and proteins into single-carbon units — molecules of carbon dioxide, which are formed by the reaction of carbon-containing food molecules with oxygen in the air.
Oceans
- The oceans are capable of both absorbing and emitting carbon dioxide. When atmospheric carbon dioxide comes into touch with ocean water, it can react with the water molecules to generate carbonic acid, a liquid form of carbon that is dissolved.
- When there is more carbonic acid in the ocean than carbon dioxide in the atmosphere, some carbonic acid may be released as carbon dioxide into the atmosphere.
- In contrast, when atmospheric carbon dioxide levels rise, more carbon dioxide will be converted to carbonic acid, and ocean acidity levels will rise.
- Some experts have expressed concern that ocean acidity is rising in some regions, possibly as a result of human activity-induced increases in atmospheric carbon dioxide.
- Although these fluctuations in ocean acidity may seem negligible by human standards, many species of marine life rely on chemical reactions that require a very specific acidity level in order to exist. In fact, ocean acidification is currently causing the demise of numerous coral reef ecosystems.
Carbon Cycle on Land
- Carbon is present in the atmosphere in the form of carbon dioxide. Natural activities such as respiration and industrial applications such as the combustion of fossil fuels release carbon into the atmosphere.
- Photosynthesis is the process through which plants absorb CO2 to generate carbohydrates. The equation looks like this: CO2 + H2O + energy → (CH2O)n +O2
- Carbon molecules are transferred from producers to consumers along the food chain. The bulk of carbon in the body resides as carbon dioxide as a result of respiration. Decomposers are responsible for consuming deceased organisms and returning the carbon from their bodies to the atmosphere. This process has the following equation: (CH2O)n +O2 → CO2 + H2O
Process of Carbon Cycle
The carbon cycle in nature consists of two primary processes:
- The conversion of oxidized form of carbon into reduced organic form by photosynthetic organisms.
- Restoration of original oxidized form through mineralization of the organic form by the micro-organisms.
1. Conversion of Oxidized form of Carbon (CO2) into Reduced Organic Form
- Photosynthesis is principally responsible for converting CO2 into organic carbon molecules.
- The most significant agents of carbon dioxide fixation are photosynthetic algae and higher plants.
- In the ocean, phytoplanktons, microscopic free-floating algae, are the dominant carbon-fixing plants. It is estimated that they fix around 1.2 x 1010 tonnes of carbon every year.
- Approximately 1.6 x 1010 tonnes of carbon are reportedly fixed annually by photosynthetic land plants.
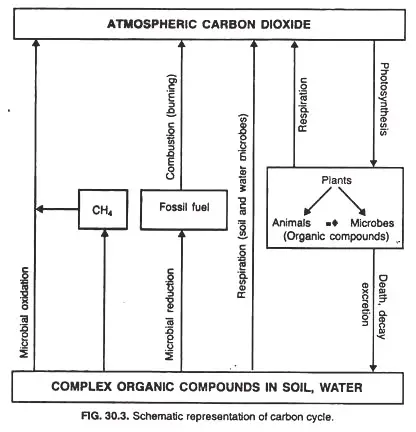
- In addition, both autotrophic and heterotrophic bacteria may produce organic matter from inorganic carbon. In addition to the presence of photosynthesis among microorganisms, these organisms also provide as an example of CO2 fixation into the following chemical compounds:
- The only source of carbon for autotrophic bacteria is carbon dioxide. In a reduction reaction, the latter fix CO2 to carbohydrates. CO2 + 2H2 → (CH2O)x + H2O
- Commonly, heterotrophic bacteria fix carbon dioxide. CH3COCOOH + CO2 → HOOCCH2. COCOOH
2. Restoration of Original Oxidized Form (CO2) through Mineralization of the Organic Form
There are three distinct mechanisms by which organic matter mineralizes and CO2 is discharged into the environment. They are:
- the respiratory process
- Unintentional (forest fire) and deliberate (fuel) combustion.
- The breakdown of organic material by microbes.
The process of respiration in plants and animals, as well as the unintentional and intentional combustion of plants and their parts, leads to the breakdown of organic carbon molecules and the subsequent release of carbon dioxide into the atmosphere.
Decomposition of Organic Matter by Microorganisms
Microorganisms, primarily bacteria and fungus, breakdown organic carbon compounds that are eventually deposited in the soil. CO2 is discharged into the atmosphere and the soil.
(i) Cellulose Decomposition
- The most prevalent organic substance in plants is cellulose. Numerous species of fungus and bacteria can easily infect it.
- The degradation of cellulose to carbon dioxide can be summarised by the following reactions:
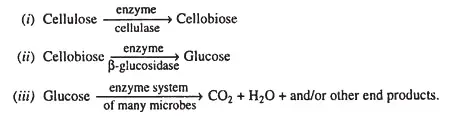
- Primarily Trichoderma, Aspergillus, Penicillium, Fusarium, Chaetomium, and Verticillium breakdown cellulose in soil. Rhizoctonia, Myrothecium, Merulius, Pleurotus, Fomes, etc.
- Bacteria such as Clostridium, Cellulomonas, Streptomyces, Cytophaga, Bacillus, Pseudomonas, Nocardia, Micromonospora, Sporocytophaga, Polyangium, Cellfalcicula, etc. are responsible for cellulose degradation in soil.
(ii) Hemicellulose Decomposition
- Hemicelluloses are the sugar polymers pentoses, hexoses, and uronic acid.
- The breakdown of hemicelluloses by bacteria is facilitated by extracellular enzymes known as hemicellulases.
- Examples of fungi that decompose hemicelluloses in soil include Chaetomium, Aspergillus, Penicillium, Trichoderma, Fusarium, and Humicola, among others. The bacteria that breakdown hemicelluloses in soil include Bacillus, Pseudomonas, Cytophaga, Vibrio, Erwinia, Streptomyces, and Actinomyces, among others.
(iii) Lignin Decomposition
- Lignin is the third most abundant plant component. It is extremely resistant to microbial decomposition.
- Nevertheless, it is known that certain fungus (Aspergillus, Penicillium, Fusarium, Lenzites, Clavaria, Polyporus, etc.) and bacteria (Streptomyces, Nocardia, Flavabacterium, Xanthomonas, Pseudomonas, Micrococcus, etc.) breakdown lignin at slow rates.
Importance of Carbon Cycle
The carbon cycle depicts the movement of carbon between the biosphere, hydrosphere, atmosphere, and geosphere of the planet. It is essential for several reasons:
- Carbon is a vital element for all life, therefore understanding its movement helps us comprehend biological processes and their influencing influences.
- Carbon dioxide, a greenhouse gas, is one type of carbon. Increased carbon dioxide levels insulate the planet, resulting in a rise in temperature. Understanding how carbon dioxide is absorbed and emitted helps us predict climate change and comprehend the climate.
- Carbon is out of balance, so it is essential to discover where it is stored and released. Carbon is not returned to the Earth at the same rate that it is deposited in living beings. Carbon is approximately 100 times more abundant in living organisms than on Earth. The combustion of fossil fuels releases vast quantities of carbon into the atmosphere and onto the planet.
- The carbon cycle is dependent on the presence of other elements and molecules. For instance, the carbon cycle is dependent on the oxygen content of the atmosphere. During photosynthesis, plants absorb carbon dioxide from the atmosphere and convert it into glucose (stored carbon) while emitting oxygen.
Application of Carbon
- It is a cost-free component with multiple applications. These include the use of diamonds or black pigment to embellish automobile rims or printer ink.
- Graphite is an additional form of carbon that has been utilised in high-temperature crucibles, arc lamp electrodes, dry cells, and pencil leads.
- Vegetal carbon is another amorphous form of carbon that is utilised as a bleaching agent and gas absorbent.
- To carbonate beverages, they utilise carbon dioxide and a fire extinguisher.
- Carbon in the solid state is referred to as dry ice.
- Carbon monoxide is also used in a variety of metallurgical reduction processes.
- In industrial solvents, carbon disulphide and carbon tetrachloride are two significant components.
Key Points on Carbon Cycle
- The carbon cycle describes the transfer of carbon between the biosphere, geosphere, hydrosphere, and atmosphere of the planet.
- Carbon is an essential component of life.
- Green plants and other photosynthetic organisms absorb atmospheric carbon dioxide and transform it into organic molecules that move through the food chain. Carbon atoms are then expelled as carbon dioxide during respiration.
- For very long periods, the creation of fossil fuels and sedimentary rocks contributes to the carbon cycle.
- The carbon cycle is related to the availability of additional chemicals.
FAQ
How Carbon Enters in the Non-Living Environment?
The nonliving environment consists of substances that have never been alive as well as carbon-containing components that persist after creatures perish. The non-living portions of the hydrosphere, atmosphere, and geosphere contain carbon as:
- Carbonate (CaCO3) rocks include coral and limestone.
- Organic stuff that has died, such as humus in soil.
- fossil fuels derived from decomposed organic materials (coal, oil, natural gas).
- Carbon dioxide in the atmosphere.
- HCO3 is formed when CO2 is dissolved with water.
How Carbon Enters Living Matter?
Carbon enters living matter via autotrophs, which are creatures capable of synthesising their own nourishment from inorganic substances.
- Photoautotrophs are responsible for the majority of carbon to organic nutrient conversion. Photoautotrophs, which are largely plants and algae, use sunlight, carbon dioxide, and water to produce organic carbon molecules (e.g., glucose).
- Chemoautotrophs are bacteria and archaea that transform carbon from carbon dioxide to an organic form, but derive their energy for the reaction from the oxidation of molecules rather than through photosynthesis.
How Carbon Is Returned to the Non-Living Environment?
Carbon is returned to the atmosphere and water by:
- Burning (as elemental carbon and various carbon molecules) (as elemental carbon and several carbon compounds).
- respiratory processes in plants and animals (as carbon dioxide, CO2).
- Decay (as carbon dioxide if oxygen is present or as methane, CH4, if oxygen is not there) (as carbon dioxide if oxygen is present or as methane, CH4, if oxygen is not present).
References
- Amelse, Jeffrey. (2020). Achieving Net Zero Carbon Dioxide by Sequestering Biomass Carbon. 10.20944/preprints202007.0576.v1.
- Carlson, C. A., Bates, N. R., Hansell, D. A., & Steinberg, D. K. (2001). Carbon Cycle. Encyclopedia of Ocean Sciences, 477–486. doi:10.1016/b978-012374473-9.00272-1
- https://www.thoughtco.com/carbon-cycle-important-607597
- https://ei.lehigh.edu/learners/cc/pdf/CarbonCycle_PrintVersion.pdf
- https://www.sciencelearn.org.nz/image_maps/3-carbon-cycle
- https://microbiologysociety.org/why-microbiology-matters/what-is-microbiology/microbes-and-the-outdoors/carbon-cycle.html
- https://www.vedantu.com/biology/carbon-cycle
- https://biologydictionary.net/carbon-cycle/
- https://www.worldatlas.com/articles/what-is-the-carbon-cycle.html
- https://dewwool.com/carbon-cycle-definitionexplanationdiagram/
- https://ugc.berkeley.edu/background-content/carbon-cycle/
- https://www.geeksforgeeks.org/carbon-cycle-definition-steps-importance-examples/
- https://en.wikipedia.org/wiki/Carbon_cycle
- https://earthobservatory.nasa.gov/features/CarbonCycle
- https://www.britannica.com/science/carbon-cycle
- https://www.noaa.gov/education/resource-collections/climate/carbon-cycle
- https://oceanservice.noaa.gov/facts/carbon-cycle.html#transcript
- https://www.khanacademy.org/science/biology/ecology/biogeochemical-cycles/a/the-carbon-cycle
- https://www.visionlearning.com/en/library/Earth-Science/6/The-Carbon-Cycle/95
- https://www.carboncyclescience.us/what-is-carbon-cycle
- https://www.lenntech.com/carbon-cycle.htm
- https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/6.6/primary/lesson/carbon-cycle-bio/
- http://www.columbia.edu/~vjd1/carbon.htm
- https://www.biologydiscussion.com/soil-microbiology/the-carbon-cycle-with-diagram-soil-microbiology/55497
- https://scied.ucar.edu/learning-zone/earth-system/biogeochemical-cycles