The Carbohydrate Fermentation Test is a biochemical test that is used to determine the ability of a microorganism to ferment a specific carbohydrate such as glucose lactose or sucrose. It is mainly applied in microbiology for identification and differentiation of bacteria based on their sugar utilization pattern. This test is referred to as Sugar Fermentation Test because it detects the fermentation of different sugars by microorganisms under controlled laboratory conditions.
It is the process in which carbohydrates are broken down by microorganisms through fermentation resulting in the production of acid or acid and gas. Different bacteria possess different enzymes and due to this reason all organisms do not ferment the same type of sugar. The test medium generally used is phenol red broth which contains the specific carbohydrate nutrient peptone and a pH indicator called phenol red.
When the organism is capable of fermenting the given sugar acid is produced which lowers the pH of the medium. Due to this decrease in pH the colour of phenol red changes from red to yellow and this indicates a positive result for acid production. In some organisms fermentation also results in gas production such as carbon dioxide or hydrogen. This gas is detected by using a small inverted glass tube placed inside the broth which is known as Durham tube and the presence of gas is observed as a bubble inside the tube.
If the organism is unable to ferment the carbohydrate the medium remains red in colour. In some cases the medium may turn deep pink or magenta due to utilization of peptone present in the medium. This process releases alkaline byproducts such as ammonia which increases the pH of the medium. Thus the carbohydrate fermentation test helps in distinguishing microorganisms based on acid production gas formation or absence of fermentation.
Objectives of Carbohydrate Fermentation Test
- To determine whether a microorganism is able to ferment a specific carbohydrate such as glucose lactose sucrose or mannitol.
- To detect the production of acid as an end product of carbohydrate fermentation by observing the colour change of pH indicator in the medium.
- To detect the production of gas during fermentation with the help of Durham tube placed in the broth.
- To differentiate and identify bacteria based on their sugar fermentation pattern such as acid production acid and gas production or no fermentation.
- To understand the enzymatic and genetic capability of microorganisms involved in carbohydrate utilization.
Principle of Carbohydrate Fermentation Test
The principle of Carbohydrate Fermentation Test is based on the ability of microorganisms to ferment a specific carbohydrate and produce acidic end products with or without gas. It is the process in which bacteria utilize sugar present in the medium by the action of specific enzymes. The test medium contains a fermentable carbohydrate peptone and a pH indicator usually phenol red which helps in detecting the change in pH during fermentation.
When the microorganism possesses the required enzymes to ferment the carbohydrate organic acids are produced as end products. These acids lower the pH of the medium and due to this decrease in pH the phenol red indicator changes its colour from red or red-orange to yellow. This colour change indicates a positive carbohydrate fermentation reaction. In some organisms fermentation is also accompanied by the production of gases such as carbon dioxide or hydrogen.
To detect gas production a small inverted glass tube known as Durham tube is placed inside the broth medium. If gas is produced during fermentation it gets collected in the Durham tube and appears as a visible bubble. On the other hand if the organism is unable to ferment the carbohydrate it utilizes the peptone present in the medium. This leads to the release of alkaline byproducts such as ammonia which increases the pH and causes the medium to remain red or turn deep pink in colour.
Requirement for Carbohydrate Fermentation Test
- Basal medium– Nutrient broth is required which contains peptone beef extract and sodium chloride. It provides basic nutrients for growth of microorganism.
- Carbohydrate substrate – A specific fermentable sugar such as glucose lactose sucrose mannitol or maltose is added to the basal medium. The carbohydrate is generally used in low concentration to avoid reversion of reaction.
- pH indicator -Phenol red is commonly used as the indicator to detect acid production. It shows colour change based on pH of the medium.
- Durham tube– A small inverted glass tube is placed inside the broth to detect gas production during fermentation.
- Test tubes– Clean sterile test tubes are required to hold the fermentation medium and Durham tube.
- Distilled water -It is used for preparation of media and dissolving the components.
- Inoculating loop or needle– It is required for aseptic transfer of test organism into the medium.
- Sterilization equipment– Autoclave is used for sterilization of media glassware and Durham tubes.
- Incubator– It is required to incubate the inoculated media at suitable temperature for microbial growth.
- Test organism– A fresh culture of microorganism to be tested is required for inoculation.
Culture Media for Carbohydrate Fermentation Test
Carbohydrate Broth– Carbohydrate broth is used as the culture medium for performing Carbohydrate Fermentation Test. It is a liquid medium that contains a basal nutrient medium along with a specific carbohydrate and a pH indicator. The composition of the medium can be modified depending on the test organism and availability of components in the laboratory.
Composition of Carbohydrate Broth Base (per 990 mL)
- Proteose peptone – 10.00 g
- HM peptone B (Beef extract) – 1.00 g
- Sodium chloride – 5.00 g
- Phenol red – 0.018 g
- (Alternatively Bromocresol purple – 0.100 g can be used as indicator)
Preparation of Carbohydrate Broth
- Required amount of basal medium components are measured and dissolved in 990 mL of distilled water.
- About 10 g of specific carbohydrate is added to the medium and mixed properly.
- The medium is heated if required to ensure complete dissolution of all components.
- About 5 to 7 mL of the broth is dispensed into clean test tubes.
- A Durham tube is placed inverted in each test tube ensuring no air bubble is present inside it.
- The tubes are loosely capped or plugged with cotton.
- Sterilization is done by autoclaving at 121°C for 15 minutes under 15 lbs pressure.
Reagents for Carbohydrate Fermentation Test
- Phenol red– It is the most commonly used pH indicator in carbohydrate fermentation test. It shows red or red-orange colour at neutral pH turns yellow at acidic pH and pink-red at alkaline pH.
- Andrade’s indicator – It appears light pink at neutral pH and changes to dark pink or red at acidic pH. At highly alkaline pH it turns yellow.
- Bromocresol purple– It is purple at neutral pH and changes to yellow under acidic conditions. In alkaline pH it retains purple colour.
- Bromothymol blue– It shows green colour at neutral pH turns yellow at acidic pH and changes to blue at alkaline pH.
- Specific carbohydrate solution -Fermentable sugars such as glucose lactose sucrose or mannitol are used as reagents for testing fermentation.
Among all these reagents phenol red is most preferred because the colour change is clearly visible non-toxic and easily available.
Carbohydrate Fermentation Test (Phenol Red Broth) – Procedure
- Fermentation broth tubes are first inspected before use. It is ensured that phenol red broth containing peptone and specific carbohydrate is clear.
- The Durham tube present inside the broth must be completely filled with liquid and free from any air bubble. If air bubble is already present, the tube is discarded or gently inverted to remove the bubble as it may give false gas production.
- Each fermentation tube is properly labelled. The name of the test organism and the carbohydrate present in the medium is written on the tube (e.g. E. coli – Glucose).
- Inoculation is carried out under aseptic conditions. A well isolated colony from a fresh culture (18–24 hours old) is picked with a sterile inoculating loop or needle. Alternatively, 1–2 drops of fresh broth culture is taken as inoculum.
- The inoculum is transferred into the fermentation broth. The tube is gently swirled to mix the contents. Care is taken not to touch the Durham tube or agitate vigorously, as this may introduce air bubbles.
- The inoculated tubes are placed in an incubator. Incubation is done at 35°C to 37°C which is suitable for most test organisms.
- The caps are kept slightly loose to allow gas exchange during incubation.
- The tubes are incubated for 18–24 hours. The results must be observed within this time period. Prolonged incubation may lead to reversion due to breakdown of peptone producing alkaline products, which may change the colour back to red or pink.
- After incubation, acid production is observed by noting colour change of the medium.
- Yellow colour indicates acid production due to carbohydrate fermentation. Red or reddish-orange colour indicates no fermentation of the carbohydrate. Pink or magenta colour indicates alkaline reaction due to peptone utilization.
- Gas production is observed by examining the Durham tube. Presence of a visible bubble in the Durham tube indicates gas production. Absence of bubble indicates no gas formation.
- An uninoculated control tube is incubated along with test tubes. This is done to check sterility and stability of the medium.
Result and Interpretation of Carbohydrate Fermentation Test
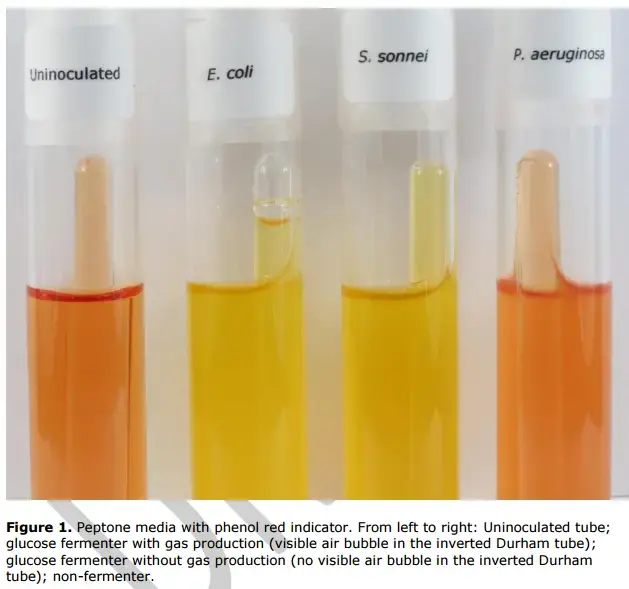
Acid production (A)– The medium turns yellow in colour. It indicates that the organism has fermented the carbohydrate and produced acidic end products.
Acid and gas production (A/G)– The medium turns yellow and a gas bubble is seen inside the Durham tube. It shows that the organism has produced both acid and gas during carbohydrate fermentation.
Negative for fermentation (-)– The medium remains red or red-orange in colour. It indicates that the organism is unable to ferment the carbohydrate.
Alkaline reaction (K)– The medium turns pink or magenta in colour. It indicates utilization of peptone with production of alkaline substances such as ammonia.
Reversion of reaction– On prolonged incubation the medium may change from yellow back to red or pink. This occurs due to exhaustion of carbohydrate and subsequent utilization of peptone by the organism.
Carbohydrate Fermentation Test – Positive and Negative Result Bacteria
Glucose (Dextrose) Fermentation
Positive (Acid and Gas production – A/G)
- Escherichia coli – Acid and gas is produced.
- Proteus vulgaris – Acid and gas is produced (H₂S may be present).
- Proteus mirabilis – Acid and gas is formed.
- Klebsiella pneumoniae – Acid and gas is produced.
- Salmonella spp. – Acid and gas is usually produced.
- Enterobacter aerogenes – Acid and gas is formed.
Positive (Acid only – A)
- Shigella spp. – Acid is produced but gas is not formed.
- Bacillus subtilis – Acid production without gas.
- Staphylococcus aureus – Acid is produced from glucose.
Negative (No fermentation – K/–)
- Pseudomonas aeruginosa – Glucose is not fermented.
- Alcaligenes faecalis – No acid or gas is produced.
Lactose Fermentation
Positive (Lactose fermenters)
- Escherichia coli – Acid and gas is produced.
- Klebsiella pneumoniae – Acid and gas is formed.
- Enterobacter aerogenes – Lactose is fermented with gas.
- Citrobacter freundii – Acid and gas is produced.
Negative (Non-lactose fermenters)
- Salmonella spp. – Lactose is not fermented.
- Shigella spp. – Lactose fermentation is absent.
- Proteus vulgaris – Negative for lactose.
- Proteus mirabilis – Lactose is not fermented.
- Pseudomonas aeruginosa – No lactose fermentation.
- Alcaligenes faecalis – Negative reaction is observed.
Sucrose Fermentation
Positive
- Proteus vulgaris – Sucrose is fermented.
- Klebsiella pneumoniae – Acid and gas is produced.
- Staphylococcus aureus – Positive for sucrose fermentation.
- Enterobacter aerogenes – Acid is formed from sucrose.
Negative
- Proteus mirabilis – Sucrose is not fermented.
- Salmonella spp. – Negative result is obtained.
- Shigella spp. – Generally non-fermenter.
Variable
- Escherichia coli – Sucrose fermentation is strain dependent.
Maltose Fermentation
Positive
- Proteus vulgaris – Maltose is fermented.
- Escherichia coli – Positive maltose reaction.
- Salmonella typhi – Maltose is fermented.
Negative
- Proteus mirabilis – Maltose is not fermented.
Mannitol Fermentation
Positive
- Shigella flexneri – Acid is produced from mannitol.
- Shigella sonnei – Mannitol is fermented.
- Salmonella spp. – Positive mannitol reaction.
- Escherichia coli – Mannitol is fermented.
- Staphylococcus aureus – Acid production is observed.
Negative
- Shigella dysenteriae – Mannitol is not fermented.
- Providencia stuartii – Negative mannitol reaction is seen.
Precautions of Carbohydrate Fermentation Test
- Durham tube should be checked properly before inoculation and must be completely filled with medium without any air bubble.
- Results should be observed within 18 to 24 hours to avoid reversion of the reaction.
- Prolonged incubation should be avoided as it may lead to alkaline reaction due to peptone utilization.
- Heat labile carbohydrates should be sterilized carefully to prevent their breakdown during autoclaving.
- Excessive shaking or agitation of tubes should be avoided as it may trap air inside Durham tube.
- While inoculating care should be taken not to touch or disturb the Durham tube.
- Gas production should be considered significant only when the medium also turns yellow.
- Pigment producing organisms should be interpreted carefully as pigment may interfere with colour of indicator.
- Proper growth of organism should be confirmed before reporting a negative result.
Uses of Carbohydrate Fermentation Test
- To differentiate bacteria based on their ability to ferment specific carbohydrates.
- To identify and classify members of Enterobacteriaceae family and other Gram-negative bacilli.
- To distinguish closely related bacterial species using their sugar fermentation pattern.
- To separate lactose fermenting organisms from non-lactose fermenting pathogenic bacteria.
- To identify yeast and other fungal species based on carbohydrate utilization.
- To study the metabolic and enzymatic capability of microorganisms.
- To detect gas producing and non-gas producing organisms during fermentation.
Advantages of Carbohydrate Fermentation Test
- It helps in differentiation and identification of microorganisms based on their carbohydrate fermentation pattern.
- It is useful in identification of Gram-negative enteric bacteria belonging to Enterobacteriaceae family.
- It helps in studying the metabolic capability of microorganisms to utilize different carbohydrates.
- It provides information about the enzymatic and genetic ability of organisms involved in sugar fermentation.
- It helps in detection of acid and gas as end products of fermentation.
- It can be performed using different types of carbohydrates such as monosaccharides disaccharides and sugar alcohols.
- It is applicable for identification of both bacteria and yeast organisms.
Limitations of Carbohydrate Fermentation Test
- Prolonged incubation may lead to reversion of reaction and give false negative result.
- It is not a confirmatory test and cannot be used alone for final identification of microorganism.
- Presence of air bubble in Durham tube may give false positive result for gas production.
- Results are time dependent and improper reading time may lead to incorrect interpretation.
- Fastidious organisms may not grow properly in the fermentation medium leading to false negative result.
- Heavy inoculum or contamination may affect the accuracy of the test result.
- A large number of carbohydrate media are required for complete identification which makes the test time consuming.
FAQ
Q1. What is a carbohydrate fermentation test?
A. Carbohydrate fermentation test is a biochemical test used to determine the ability of bacteria to ferment specific carbohydrate and produce acid or acid with gas as end products.
Q2. What is the principle of the carbohydrate fermentation test?
A. It is based on the ability of microorganisms to ferment carbohydrate sugars resulting in production of acid and sometimes gas which changes the colour of the medium due to pH indicator.
Q3. How is a carbohydrate fermentation test performed?
A. The test is performed by inoculating bacteria into carbohydrate broth containing pH indicator and Durham tube and then incubated at suitable temperature for 18–24 hours.
Q4. How do you interpret the results of a carbohydrate fermentation test?
A. The result is interpreted based on colour change of medium and presence or absence of gas in Durham tube after incubation.
Q5. What is the purpose of the Durham tube in a fermentation test?
A. Durham tube is used to detect gas production during carbohydrate fermentation.
Q6. What pH indicator is used in carbohydrate fermentation tests?
A. Phenol red is commonly used as pH indicator in carbohydrate fermentation test.
Q7. What are the uses of the carbohydrate fermentation test?
A.
- It is used to differentiate bacteria based on sugar fermentation.
- It is used for identification of enteric bacteria.
- It is helpful in routine laboratory diagnosis.
Q8. What carbohydrates are commonly used in fermentation tests?
A. Commonly used carbohydrates are glucose, lactose, sucrose, maltose and mannitol.
Q9. What are the possible results of a carbohydrate fermentation test?
A.
- Acid production only.
- Acid and gas production.
- No fermentation (alkaline reaction).
Q10. What does a positive result in a carbohydrate fermentation test indicate?
A. Positive result indicates that the organism is able to ferment the carbohydrate producing acid with or without gas.
Q11. What does a negative result in a carbohydrate fermentation test indicate?
A. Negative result indicates that the organism is unable to ferment the carbohydrate and peptone is utilised producing alkaline reaction.
Q12. What are the end products of carbohydrate fermentation?
A. End products are organic acids such as lactic acid, acetic acid and sometimes gas like carbon dioxide and hydrogen.
Q13. Why is carbohydrate fermentation important in microbiology?
A. It is important for identification and differentiation of bacteria based on their metabolic properties.
Q14. How do bacteria ferment carbohydrates?
A. Bacteria ferment carbohydrates by enzymatic breakdown of sugars through glycolytic pathway producing acids and gases.
Q15. What is the role of peptone in carbohydrate fermentation media?
A. Peptone acts as nitrogen source and if carbohydrate is not fermented it is utilised resulting in alkaline by-products.
- American Society for Microbiology. (2012, November 1). Protocols: Carbohydrate fermentation by bacteria. https://asm.org/protocols/carbohydrate-fermentation-protocol
- Aryal, S. (2022, August 10). Fermentation test – Principle, procedure, uses and interpretation. Microbiology Info.com. https://microbiologyinfo.com/fermentation-test/
- Aryal, S. (2022, August 10). Phenol red fermentation test – Principle, procedure, uses and interpretation. Microbiology Info.com. https://microbiologyinfo.com/phenol-red-fermentation-test/
- Cooper, C. R., Jr. (2019). Carbohydrate fermentation test. Youngstown State University. http://crcooper01.people.ysu.edu/microlab/fermentation.pdf
- Dahal, P. (2023, May 23). Carbohydrate fermentation test (sugar fermentation test). Microbe Notes. https://microbenotes.com/carbohydrate-fermentation-test/
- Dahal, P. (2023, March 6). OF test- Oxidation/oxidative-fermentation/fermentative test. Microbe Notes. https://microbenotes.com/oxidation-fermentation-of-test/
- DrChika. (2022, December 28). Sugar (glucose) utilization test. Microbiology Class. https://microbiologyclass.net/sugar-glucose-utilization-test/
- Expert Analysis of the Carbohydrate Fermentation Test for Microbial Identification. (n.d.). [Unpublished expert analysis provided in source text].
- Filo. (2025, October 9). Following a fermentation test, the phenol red media turns pink. What is the reason? https://askfilo.com/user-question-answers-smart-solutions/following-a-fermentation-test-the-phenol-red-media-turns-3430343036303538
- Hackmann, T. J. (2024). The vast landscape of carbohydrate fermentation in prokaryotes. FEMS Microbiology Reviews, 48(4), fuae016. https://doi.org/10.1093/femsre/fuae016
- Hartline, R. (2023, February 18). 1.22: Fermentation. Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.22%3A_Fermentation
- Hartline, R. (2023, February 18). 1.27: MR-VP tests. Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.27%3A_MR-VP_Tests
- Life in the Lab Staff. (2025, October 27). The essential guide to phenol red in cell culture media. Thermo Fisher Scientific. https://www.thermofisher.com/blog/life-in-the-lab/the-essential-guide-to-phenol-red-in-cell-culture-media/
- Lipscomb, G. L., Schut, G. J., Thorgersen, M. P., Nixon, W. J., Kelly, R. M., & Adams, M. W. W. (2014). Engineering hydrogen gas production from formate in a hyperthermophile by heterologous production of an 18-subunit membrane-bound complex. Journal of Biological Chemistry, 289(5), 2873–2879. https://doi.org/10.1074/jbc.M113.530725
- MacKenzie, E. (2025, October 15). 31.4: Phenol red fermentation test. Biology LibreTexts. https://bio.libretexts.org/Courses/Irvine_Valley_College/IVC_Microbiology_Lab_Manual/31%3A_METABOLIC_TESTING/31.04%3A_Phenol_Red_Fermentation_Test
- MacKenzie, E. (2025, October 15). 31.5: Methyl red / Voges-Proskauer (MR/VP) test. Biology LibreTexts. https://bio.libretexts.org/Courses/Irvine_Valley_College/IVC_Microbiology_Lab_Manual/31%3A_METABOLIC_TESTING/31.05%3A_Methyl_Red___Voges-Proskauer_(MR_VP)_Test
- Reiner, K. (2012, November 1). Carbohydrate fermentation protocol. American Society for Microbiology. https://asm.org/protocols/carbohydrate-fermentation-protocol
- Reiner, K. (2012, November 1). Fermentation [Image gallery]. American Society for Microbiology. https://asm.org/image-gallery/fermentation
- Remel. (2010). Yeast fermentation broth w/ brom thymol blue and Durham tube [Instructions for use]. Thermo Fisher Scientific. https://documents.thermofisher.com/TFS-Assets/LSG/manuals/IFU65300.pdf
- Taylor & Francis. (n.d.). Mixed acid fermentation. Taylor & Francis Knowledge. https://taylorandfrancis.com/knowledge/Medicine_and_healthcare/Pharmaceutical_medicine/Mixed_acid_fermentation/
- The Ohio State University. (n.d.). Flow chart of Gram negative organisms. https://medicine.osu.edu/-/media/files/medicine/departments/pharmacy/asp/clinical-microbiology/gramnegorganisms.pdf
- UK Health Security Agency. (2025, July 31). UK standards for microbiology investigations: Identification of Shigella species (ID 20, Issue 4.1). https://www.gov.uk/government/publications/smi-id-20-identification-of-shigella-species
- U.S. Food and Drug Administration. (2017, November 13). BAM media M121: Phenol red carbohydrate broth. https://www.fda.gov/food/laboratory-methods-food/bam-media-m121-phenol-red-carbohydrate-broth
- VUMIE. (2022, June 16). Gas from glucose. https://vumicro.com/help/content/gas_from_glucose.htm
- VUMIE. (2022, June 16). Phenol red glucose (dextrose) broth with Durham tube. https://vumicro.com/docs/phenol-red-glucose-dextrose-broth-with-durham-tube/
- VUMIE. (2022, November 1). Sucrose fermentation test. https://vumicro.com/docs/sucrose-fermentation-test/
- Wikipedia. (2024, March 7). Ethanol fermentation. https://en.wikipedia.org/wiki/Ethanol_fermentation
- Wikipedia. (2024, February 28). TSI slant. https://en.wikipedia.org/wiki/TSI_slant