Brucella is a genus of small Gram-negative coccobacilli. It is non-motile and non-spore forming, and it is the organism responsible for a zoonotic disease called brucellosis. It is the process where the bacteria survive as facultative intracellular parasite inside different host cells. These organisms are named after David Bruce and are placed under the family Brucellaceae.
Brucella species enter the body mainly through contact with infected animals or by consumption of contaminated dairy products. The major source of infection in humans is unpasteurized milk. It is the causative agent of Malta fever or undulant fever. In livestock the infection is associated with abortion, infertility and swelling of reproductive organs. In humans it results in acute febrile illness with headache, weakness and joint pain.
The pathogenicity of Brucella is controlled by its ability to live and multiply inside phagocytic cells. After entry, the organism is enclosed in a membrane-bound compartment called Brucella containing vacuole (BCV). This vacuole avoids lysosomal destruction and slowly changes into an endoplasmic reticulum derived compartment where the bacteria replicate. This process is referred to as intracellular survival and it is mediated by a Type IV secretion system which releases different effector proteins into the host cell.
Some of the important species infecting humans are B. melitensis, B. abortus, B. suis and B. canis. These are the common agents responsible for disease in different animals and humans.
Scientific classification of Brucella
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Alphaproteobacteria |
| Order: | Hyphomicrobiales |
| Family: | Brucellaceae |
| Genus: | Brucella Meyer and Shaw 1920 (Approved Lists 1980) |
Characteristics of Brucella
- It is a Gram-negative coccobacilli which appear as very small short rods.
- It is non-motile and non-spore forming, and the cells are usually arranged singly or sometimes in pairs.
- The organisms show weak acid-fast property when stained by Stamp’s modification of Ziehl–Neelsen method.
- The genus is placed under the family Brucellaceae and belongs to the order Rhizobiales.
- These species show high genetic similarity and most of them have two circular chromosomes with high GC content.
- It is strictly aerobic in nature and the growth is slow on culture media, taking several days for colony formation.
- Some of the species grow better when the atmosphere contains 5–10% CO₂.
- Oxidase and urease tests are positive in most species. Urease helps in survival during the passage through acidic environments.
- Different species can be differentiated by H₂S production, reaction with aniline dyes and by phage lysis.
- It is a facultative intracellular parasite and mainly infects phagocytic cells like macrophages.
- The organisms also show preference for placental tissues because these tissues contain erythritol which helps in growth.
- It does not produce classical toxins, but virulence is controlled by its lipopolysaccharide (LPS) and the Type IV secretion system.
- Smooth LPS strains show full virulence, while rough strains are less virulent.
- The bacteria survive in a vacuole called Brucella containing vacuole (BCV) which avoids lysosomal fusion.
- The BCV later associates with endoplasmic reticulum membranes forming a compartment suitable for multiplication.
- It can survive for long periods in cool moist environments, in contaminated soil, milk or aborted materials.
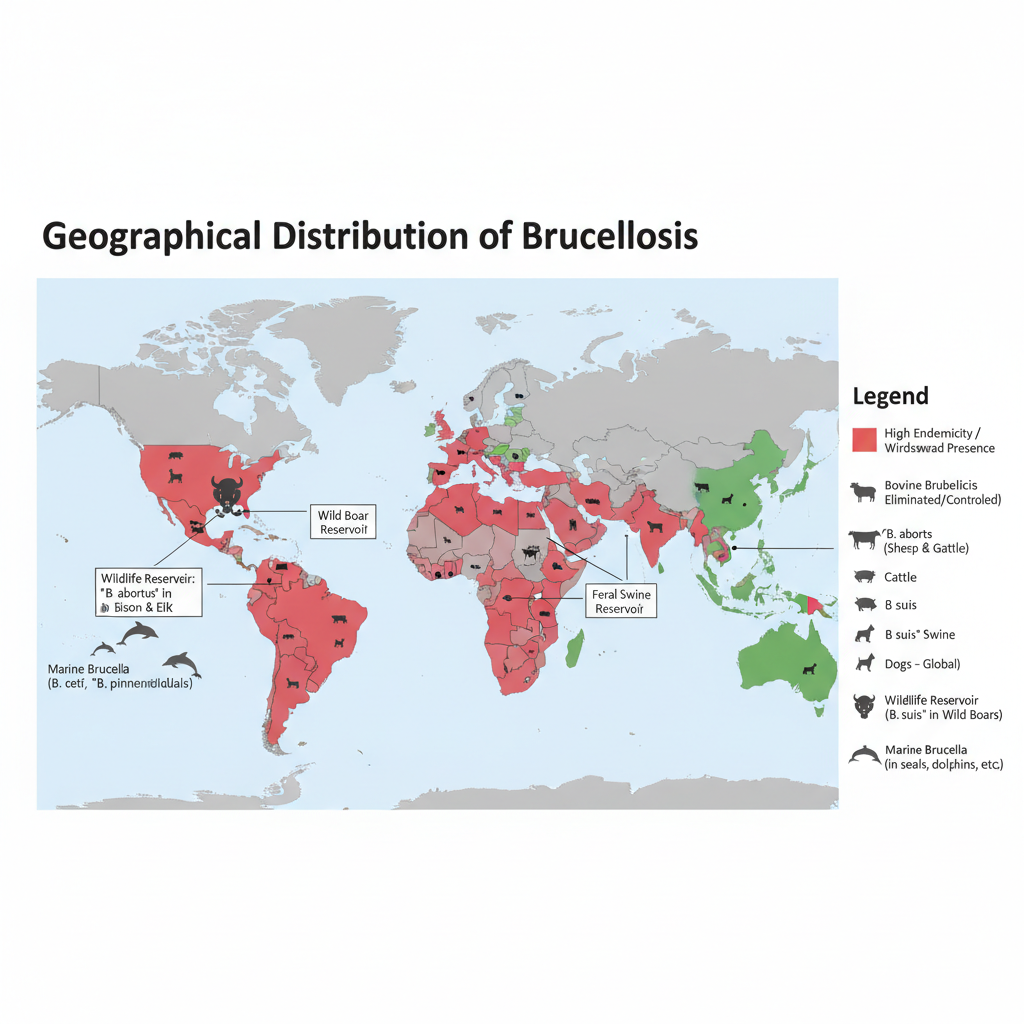
Geographical Distribution of Brucellosis
Brucellosis is present in many parts of the world and it is regarded as an important zoonotic disease. It is the process where the infection remains common in regions having poor control measures in livestock. The human cases are still reported in large numbers every year.
- It is widely distributed in the Mediterranean Basin including Portugal, Spain, Greece, Turkey and different parts of North Africa.
- The disease is also common in the Middle East and countries around the Arabian Gulf.
- Central and Southwest Asia show increasing reports of infection in recent years.
- In Latin America and different parts of South and Central America the disease remains endemic.
- Mexico continues to report cases, while in the United States the infection is uncommon but B. suis occurs in hunters handling feral swine.
- It is endemic in sub-Saharan Africa and in different Asian regions like China, India and the Indian subcontinent.
- B. melitensis, which infect sheep and goats, is absent in some regions like North America, North Europe, South-East Asia and Oceania.
- B. suis is more common in South America and South-East Asia, and a specific biovar occurs from Scandinavia to the Balkans in wild boars.
- B. abortus is present in many African, European, Asian and American countries, and a known reservoir exists in the Greater Yellowstone Ecosystem infecting bison and elk.
- B. canis is distributed globally through dogs and causes generally mild human infection.
- Some developed countries have eliminated bovine brucellosis, like Canada, Japan, northern Europe and Australasia.
- Wildlife reservoirs such as infected bison, elk and feral swine still maintain the organism in the environment complicating full eradication.
- Marine mammals like dolphins and seals may be infected by species such as B. ceti and B. pinnipedialis in different oceans.
Habitat of Brucella
Brucella is a facultative intracellular parasite and its main habitat is inside the host cells. It survives for long time inside different mammalian tissues and also persists outside the host under suitable conditions.
- It mainly infects phagocytic cells like macrophages and dendritic cells, and the organisms can also multiply in epithelial cells, fibroblasts and endothelial cells.
- After entry, the bacteria remain inside a membrane-bound structure called Brucella containing vacuole (BCV).
- The BCV slowly changes into an endoplasmic reticulum (ER) derived compartment where the multiplication takes place.
- Brucella shows strong tropism towards organs rich in reticuloendothelial cells such as spleen, liver, lymph nodes and bone marrow.
- In the bone marrow it is found inside granulocytes, monocytes and progenitor cells and it may persist for longer duration.
- In reproductive organs the organisms infect placental trophoblasts in pregnant animals and the testis or epididymis in males.
- The presence of erythritol in reproductive tissues of ruminants promotes the growth of the bacteria.
- It may also localize in mammary gland, joints and sometimes in adipose tissues.
- Even though it is an intracellular bacterium, it can survive for months in cool moist external environment.
- Aborted fetuses, placental materials, milk, urine, semen, and dung act as temporary habitats for the organisms.
- Soil acts as another environmental source and some species like B. microti are isolated from soil samples.
- Different wildlife species act as natural reservoirs such as bison, elk, wild boars, hares and marine mammals including dolphins and seals.
Classification of Brucella
- Domain: Bacteria
- Phylum: Proteobacteria
- Class: Alphaproteobacteria
- Order: Rhizobiales
- Family: Brucellaceae
- Genus: Brucella
Species:
- B. melitensis – Sheep, goats, humans
- B. abortus – Cattle, buffalo, humans
- B. suis – Swine, humans
- B. canis – Dogs, foxes, coyotes, humans
- B. ovis – Rams
- B. neotomae – Desert wood rats
- B. ceti – Dolphins, porpoises
- B. pinnipedialis – Seals, sea lions
- B. microti – Field voles
- B. inopinata – Humans (rare)
- B. anthropi – Humans (formerly Ochrobactrum anthropi)
- B. vulpis – Foxes
The genus Brucella comprises both classical and novel species, with some formerly classified under the genus Ochrobactrum now included based on genetic analyses.
Morphology of Brucella
Brucella shows a very typical morphology and the cells appear as small Gram-negative coccobacilli. The organism is short rod shaped and the size usually ranges between 0.6–1.5 µm in length and 0.5–0.7 µm in width.
- The cells are mostly seen singly but sometimes they appear in pairs or in small groups.
- It is non-motile and no flagella are present, and the organism does not form spores or capsules.
- The basic shape remains constant, although pleomorphic forms may be seen in old cultures.
- It stains as Gram-negative and does not show bipolar staining.
- The cells resist decolourisation by weak acids and therefore stain red in Stamp’s modification of Ziehl–Neelsen method, so it is weakly acid-fast.
- The outer membrane contains lipopolysaccharide (LPS) and the species appear as smooth or rough depending on the O-side chain.
- Smooth strains form round translucent pearly colonies, while rough strains form dull granular colonies.
- The organism contains two circular chromosomes of different sizes.
- It shows unipolar type of growth which is similar to other Alphaproteobacteria.
Human Infections Caused by Brucella Species
Human brucellosis is a zoonotic infection and it appears as an acute febrile illness or a flu-like condition. It is the process where the organisms enter the human body from infected animals or their products. The disease is also known as undulant fever or Malta fever.
Causative Species
- B. melitensis is the most virulent and the most common cause of human infection. It mainly comes from sheep and goats.
- B. suis infects humans from swine and the virulence is intermediate.
- B. abortus is usually less severe and more of the cases are subclinical.
- B. canis produces milder disease in humans and is transmitted from dogs.
- Some other species like B. ceti, B. pinnipedialis, B. neotomae and B. inopinata may produce moderate infection in humans.
Clinical Features
- It has a long incubation period and the symptoms are non-specific.
- Fever is undulating or intermittent and occurs commonly in most patients.
- There is weakness, headache, chills and profuse sweating.
- Joint pain, anorexia and weight loss are also common.
Complications
- Osteoarticular problems like arthritis, spondylitis and osteomyelitis are the common complications.
- Endocarditis occurs less often but it is the major cause of death.
- The organisms localize in liver and spleen causing enlargement of these organs.
- Abscess formation may be seen mainly in B. suis infection.
- Infection may involve the testis or epididymis.
- Neurological involvement occurs in some cases as neurobrucellosis.
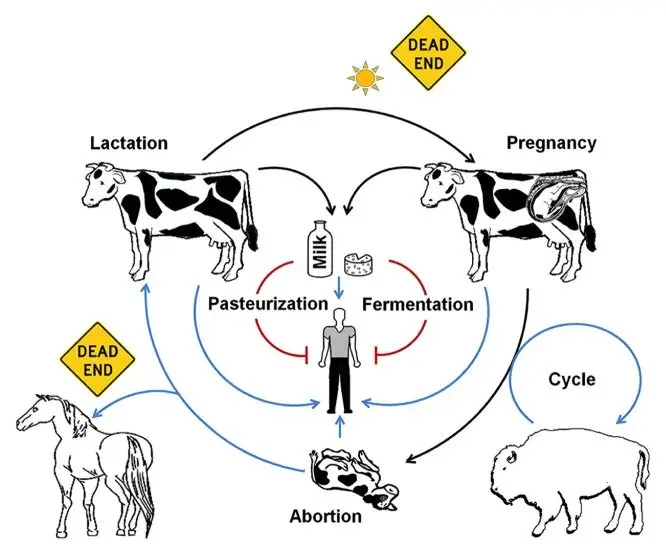
Transmission to Humans
- Ingestion of unpasteurized milk and fresh soft cheeses is the main mode of transmission to the general population.
- Direct contact with infected animals or their tissues through cuts or mucosal surfaces can transmit the organisms.
- Inhalation of aerosols is important in laboratories and industrial settings.
- Farmers, veterinarians and abattoir workers are at higher risk of exposure.
- Hunters handling feral swine are mainly associated with B. suis infection.
- Laboratory exposure is common because very few organisms are sufficient to cause infection.
Rare Modes
- Person-to-person spread is very rare.
- Transmission may occur during sexual intercourse.
- Vertical transmission from infected mother may occur during pregnancy or through breast milk.
- It may also spread through tissue transplantation or blood transfusion in rare cases.
Culture Media for Brucella
Brucella is fastidious in nature and the growth is slow. It is the process where enriched media and special incubation conditions are needed for successful isolation.
Basal and Enriched Media
- Different basal media like Brucella medium base, tryptose soy agar and blood agar base can support growth.
- Serum (2–5%) from bovine or equine source is often added to improve growth especially for fastidious strains.
- Serum–dextrose agar (SDA) is used commonly for observing colony characters.
- Plates are incubated at 35–37°C under aerobic conditions and usually with 5–10% CO₂ which supports growth of all species.
- Colonies appear slowly, mostly after 3–4 days, but plates should be kept for 7–10 days before reporting negative.
Special Media for Clinical Samples
- Castañeda’s biphasic medium is used for blood, body fluids and milk. It contains both liquid and solid phases in the same bottle.
- The bottle is incubated at 37°C and kept in a slanted position to allow contact with both phases.
- TUMS medium is a similar system but contains urea agar on the slant and brain heart infusion in the liquid phase, helping in early detection due to urease activity.
- Automated blood culture systems like Bactec or BacT/Alert detect CO₂ production and can identify growth within 5–7 days.
Selective Media
- Selective media are used when samples contain other contaminating organisms.
- Farrell’s medium and modified Thayer–Martin medium are used for selective isolation.
- Antibiotic supplements containing polymyxin B, bacitracin, natamycin, nalidixic acid, nystatin and vancomycin can be added to Columbia agar with serum for selective growth.
- Modified Brucella selective (MBS) medium is used mainly for selecting B. abortus strains.
- Vaccine strains show different growth patterns; RB51 grows in presence of rifampicin, while Rev.1 does not grow in presence of basic fuchsin or thionin.
Biochemical Test of Brucella melitensis
The organism shows a typical pattern of biochemical reactions which helps in its identification. It is the process where simple enzyme tests and dye sensitivity reactions are used.
- In Gram staining the cells appear as small Gram-negative coccobacilli.
- It is non-motile and no spores are produced.
- Capsule formation is absent.
- Oxidase test is positive.
- Catalase test is positive.
- Urease is positive and the hydrolysis is detected within 2 hours.
- Hydrogen sulphide (H₂S) production is negative.
- It grows on thionin (0.002%) but no growth occurs at 0.004%.
- It grows on basic fuchsin (0.002%) but not at higher concentration (0.004%).
- CO₂ is not required for growth.
- The organism is not destroyed by Tbilisi phage.
- Good growth occurs on Brucella agar within 48 hours at 37°C.
- Colonies appear smooth, glistening and pin-point.
- On Gram stain from colonies, it again appears as small Gram-negative coccobacilli.
- It may sometimes be misidentified as Moraxella phenylpyruvica in API 20NE system.
Differential Features of Brucella Species
| Species | Natural Hosts | Urease | H₂S Production | Dye Sensitivity | Phage Sensitivity | CO₂ Requirement | Growth on MacConkey Agar | Colony Morphology |
|---|---|---|---|---|---|---|---|---|
| B. abortus | Cattle, buffaloes | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. melitensis | Sheep, goats, camels | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. suis | Pigs, wild boar, hares, reindeer | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. canis | Dogs | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. ovis | Sheep | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. neotomae | Desert woodrats | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. microti | Voles | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. pinnipedialis | Seals, sea lions, walruses | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. ceti | Dolphins, porpoises | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. intermedia | Rarely in humans | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. anthropi | Rarely in humans | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
Biotypes and Phage Types of Brucella Species
Brucella species show different biovars which help in field identification and studying the distribution of strains. It is the process where biochemical patterns, dye reactions and phage sensitivity are used for typing.
Biotypes of Brucella
- Brucella abortus shows biovars 1–7 and 9. These are mainly associated with cattle, buffaloes and bison.
- Biovar-3 of B. abortus is common in Nigeria and shows a regional distribution.
- Brucella melitensis has biovars 1–3, and biovar-1 is regarded as the most pathogenic for humans, mostly linked to sheep and goats.
- Brucella suis shows biovars 1–5. Biovar-2 infects wild boar and hare in Europe and forms important wildlife reservoirs.
- Biovar-5 of B. suis is rare and isolated from rodents in Northern Caucasus and South-West Siberia, showing limited regional circulation.
- Brucella canis is considered a stable rough-mutant of B. suis biovar-1 and it causes canine brucellosis in kennels.
Phage Types of Brucella
- Tbilisi (Tb) phage is used for typing B. abortus and B. melitensis. It was isolated in 1955.
- Weybridge (Wb) phage also acts on B. abortus and B. melitensis and is used to distinguish laboratory strains.
- Firenze (Fi) phage is used in a similar way but its activity may vary with strains showing rough tendencies.
- Berkeley-2 (Bk2) phage is used for classical smooth species and is important for epidemiologic investigations.
- Izatnagar-1 (Iz1) phage is applied to rough Brucella strains which do not react with standard phage sets.
- Rough-specific phages (R/C) are used mainly for R-type strains and the simple R-phage is also applied for similar work.
Cell Wall Components and Antigenic Structure of Brucella Species
Brucella are small Gram-negative coccobacilli. These cells are non-motile and no true capsule or spore is produced. The outer cell membrane is similar to other Gram-negative organisms but the major component is LPS. It is the presence of this LPS that gives many of the antigenic features.
The LPS of Brucella is considered unique. It is the process where the lipid A, core polysaccharide and O-side chain together form the complete LPS, but the toxicity remains very low. It is less pyrogenic and this reduced endotoxic activity helps the organism to resist early immune recognition.
In this structure, the lipid A contains diaminoglucose instead of glucosamine and it also have longer acyl chains (C28). These features is responsible for weak activation of TLR4. The core is connected mainly by amide linkages.
Antigenic Types (Smooth and Rough)
Antigenic variation is mainly due to the presence or absence of the O-side chain of LPS.
- Smooth (S) strains –
These strains possess the complete O-polysaccharide. The S-LPS is important for full virulence because it protects the organism from lytic substances of host cells. It is also involved in avoiding apoptosis of host cells. The O-chain is formed of 4-formamido-4,6-dideoxymannose. Species like B. melitensis, B. abortus and B. suis are smooth. - Rough (R) strains –
These strains lack the O-chain or it is very reduced. Such cells are more easily removed by the immune system. B. canis and B. ovis are naturally rough. It is the process where smooth cultures sometimes undergo dissociation and produce rough colonies.
Specific Antigenic Epitopes
The antigenic activity of smooth Brucella is due to the O-polysaccharide side chains. These chains carry the A and M antigens.
- A antigen and M antigen –
Both antigens are homopolymers of 4,6-dideoxy-4-formamido-D-mannopyranose. In A antigen the linkage is always 2-1. In M antigen every fifth linkage is 3-1.
B. abortus is A-dominant and B. melitensis is M-dominant. - Cross-reactivity –
The O-LPS is largely shared among Brucella species, so it is difficult to separate them serologically. It also cross-reacts with Yersinia enterocolitica O:9 because the structure of O-antigen is almost identical. This is referred to as the cause of false-positive reactions in many tests.
Other Cell Envelope Components
- Outer Membrane Proteins (OMPs)– These proteins are grouped into several classes. Some important genes are omp25, omp2a, omp2b, omp31 and omp31b. They are used to differentiate species by methods like RFLP. It is the presence of these proteins that take part in host–pathogen interaction.
- Cyclic β-1,2-glucans (CβG)– These are periplasmic glucans. This compound is involved in controlling the phagosome–lysosome fusion. It modifies the Brucella Containing Vacuole (BCV) membrane surface. This is referred to as an important virulence factor.
- BvrR/BvrS System– This is a two-component regulatory system. It is the process that controls early interaction of the bacterium with host cells. It regulates the expression of OMPs like Omp3a (Omp25a) and Omp3b (Omp22). Mutants in this system show altered LPS structure. It is important for membrane homeostasis.
Virulence Factors of Brucella Species
It is the virulence property of Brucella species which is mainly based on its ability to live and multiply inside host phagocytic cells. These organisms do not produce the common virulence structures like toxins or fimbriae, but instead depend on several regulated systems that help in intracellular survival.
Type IV Secretion System (T4SS)
The major virulence system is the Type IV Secretion System. It is encoded by the virB operon. It is the process where effector proteins are transferred from the Brucella Containing Vacuole (BCV) to the host cytoplasm.
This system is required for the formation of the replicative BCV that is derived from the endoplasmic reticulum. When the T4SS is not functional, the BCV fuses with lysosomes and the bacteria is destroyed.
Some of the main effector proteins are–
– BtpA (Btp1) and TcpB. These proteins reduce dendritic cell activation. TcpB also helps intracellular replication by inducing Unfolded Protein Response genes.
– BtpB. This protein interacts with MyD88 and blocks TLR signaling (TLR2, TLR4, TLR5 and TLR9).
– VceC. It is reported to induce inflammation by UPR-dependent NF-κB activation.
– RicA. It interacts with Rab2 and helps in BCV traffic.
– SepA. It is important in early survival by avoiding LAMP1 fusion.
– BPE123. It targets ENO-1 and helps in intracellular survival.
– BPE005. It induces collagen deposition and reduces matrix metalloproteinase 9.
Lipopolysaccharide (LPS)
LPS is another essential virulence factor. The LPS of Brucella is less toxic. It is weak in pyrogenicity and helps the organism to avoid early immune detection.
The elongated fatty acids (C28) present in lipid A cause poor activation of TLR4. Smooth LPS (S-LPS) in B. melitensis, B. abortus and B. suis is important for full virulence. It is the process where the S-LPS prevents host cell apoptosis and protects the organism from lytic substances.
It also takes part in entry to macrophages by interacting with lipid rafts.
Regulatory and Metabolic Factors
BvrR/BvrS Two-Component System
This system helps in early interaction with the host cell surface. It regulates membrane homeostasis and several envelope proteins like Omp3a and Omp3b. It also influences the VirB system through VjbR.
Cyclic β-1,2-glucan (CβG)
These glucans are important for intracellular survival. It is the factor that controls fusion of BCV with lysosomes by affecting cholesterol release inside the phagosome.
Resistance to Oxidative Stress
The enzyme exonuclease III encoded by xthA-1 is used in base excision repair. It protects against oxidative damage by reactive oxygen species.
Erythritol
This is a sugar alcohol found in reproductive tissues of animals. It is the major growth factor that allows heavy multiplication of Brucella in pregnant ruminants and pigs.
Motility Evasion
Even though Brucella is non-motile, the flagellin domain that activates TLR5 is absent. It is helpful for avoiding TLR5 recognition.
Pathogenesis of Brucellosis
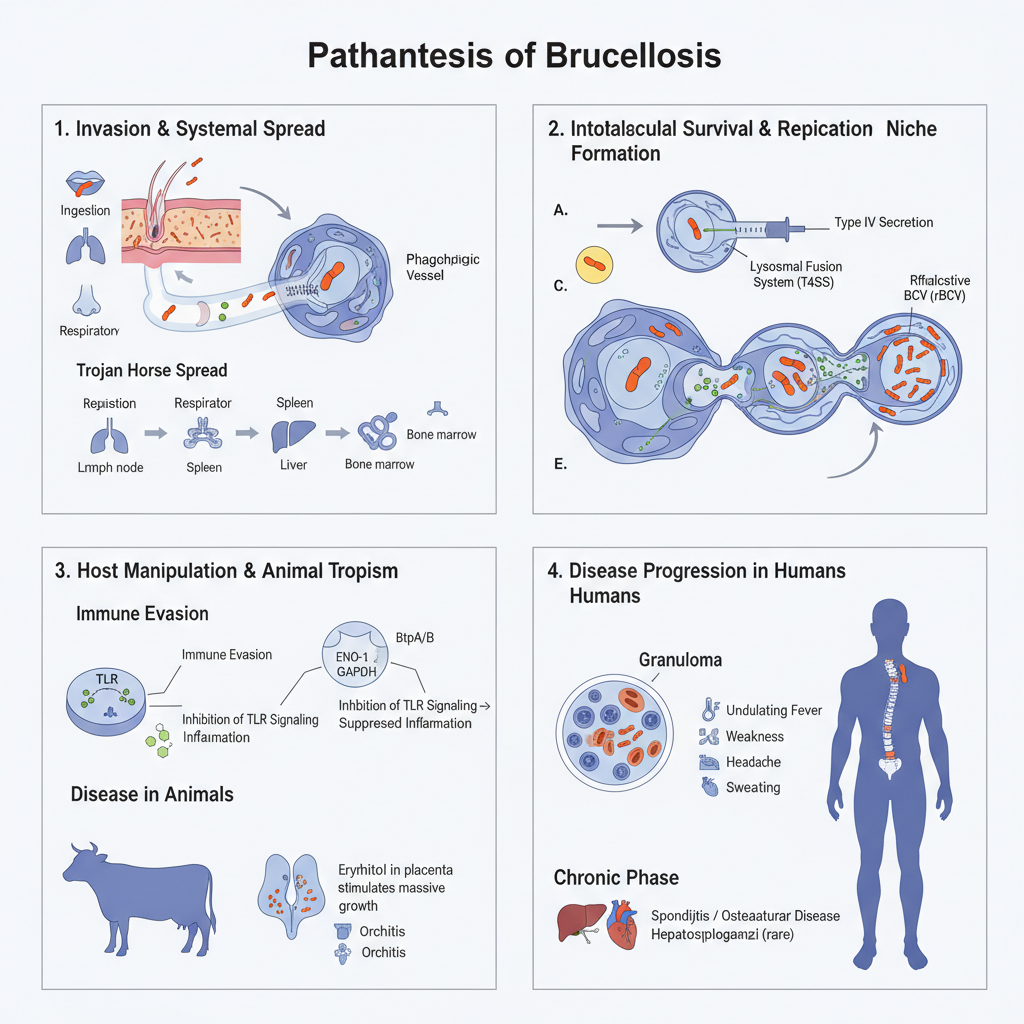
1. Invasion and Initial Systemic Spread
- Brucella is a facultative intracellular pathogen and it enter the host mainly through ingestion, conjunctiva, respiratory tract, and abraded skin.
- It is the mouth and the respiratory aerosols that act as major route in animals and humans.
- After crossing the mucosal lining, the organisms is phagocytosed by macrophages and dendritic cells.
- These cells is the preferred host cells and Brucella can also replicate in epithelial cells, fibroblasts, microglia and endothelial cells.
- The invasion in many smooth strains occur by a zipper-like mechanism where it is mediated by lipid rafts on macrophage membrane.
- Entry inside non-phagocytic cells require actin polymerisation and it is the Rho, Rac and Cdc42 proteins involved in this step.
- The organisms use the mononuclear phagocytes as Trojan horse and spread to lymph nodes, spleen, liver and bone marrow.
- Bone marrow is the major reservoir where B. abortus remain for long time within granulocytes, monocytes and progenitor cells.
2. Intracellular Survival and Replication Niche
- Brucella does not produce classical exotoxins. It is the intracellular survival that define its virulence.
- After internalisation, the organisms reside inside Brucella Containing Vacuole (BCV).
- BCV avoid lysosomal fusion and follow an altered trafficking pathway.
- The virB operon encode Type IV Secretion System (T4SS) which is the major virulence determinant.
- T4SS is used to inject effector proteins inside host cytoplasm and helps the BCV to escape destruction.
- Strains lacking functional T4SS is unable to avoid lysosomal fusion.
- The BCV then gradually acquire endoplasmic reticulum (ER) markers like calnexin and calreticulin.
- This ER-derived compartment is referred to as rBCV and it is the actual replicative niche of Brucella.
3. Molecular Mechanisms of Host Manipulation
- It is several effector proteins that help in immune evasion.
- BtpA/BtpB proteins having TIR domain interact with MyD88 and inhibit TLR signalling (TLR2, TLR4, TLR5, TLR9).
- This suppress the maturation of dendritic cells and reduce inflammatory response.
- Smooth LPS of Brucella is less endotoxic and it poorly activate TLR4, allowing a stealth infection.
- LPS also prevent apoptosis of infected cells helping prolonged survival.
- Metabolic manipulation occur through BPE123 which activate alpha-enolase (ENO-1) to support glycolytic flow near BCV.
- GAPDH is also recruited to BCV and both enzymes support replication.
- Alternative activated macrophages (AAMs) provide high glucose environment which favour chronic infection.
- Tissue modification in liver is influenced by BPE005 which induce collagen deposition and reduce MMP-9 activity.
4. Disease Progression in Hosts
In Animals
- The organisms show special tropism for reproductive organs in animals.
- Infection cause abortion, infertility, orchitis, epididymitis and retained placenta.
- Erythritol present in placenta and genital tissues stimulate massive growth of Brucella.
In Humans
- It is a systemic infection involving several organs.
- Minute granulomas made of epithelioid cells, PMNs, lymphocytes and giant cells is formed.
- Many symptoms arise due to hypersensitivity to brucellar antigens.
Clinical Features
- Acute phase – undulating fever, weakness, headache, arthralgia and sweating. Incubation period may be long.
- Chronic phase – osteoarticular disease, spondylitis, hepatomegaly, splenomegaly and sometimes endocarditis.
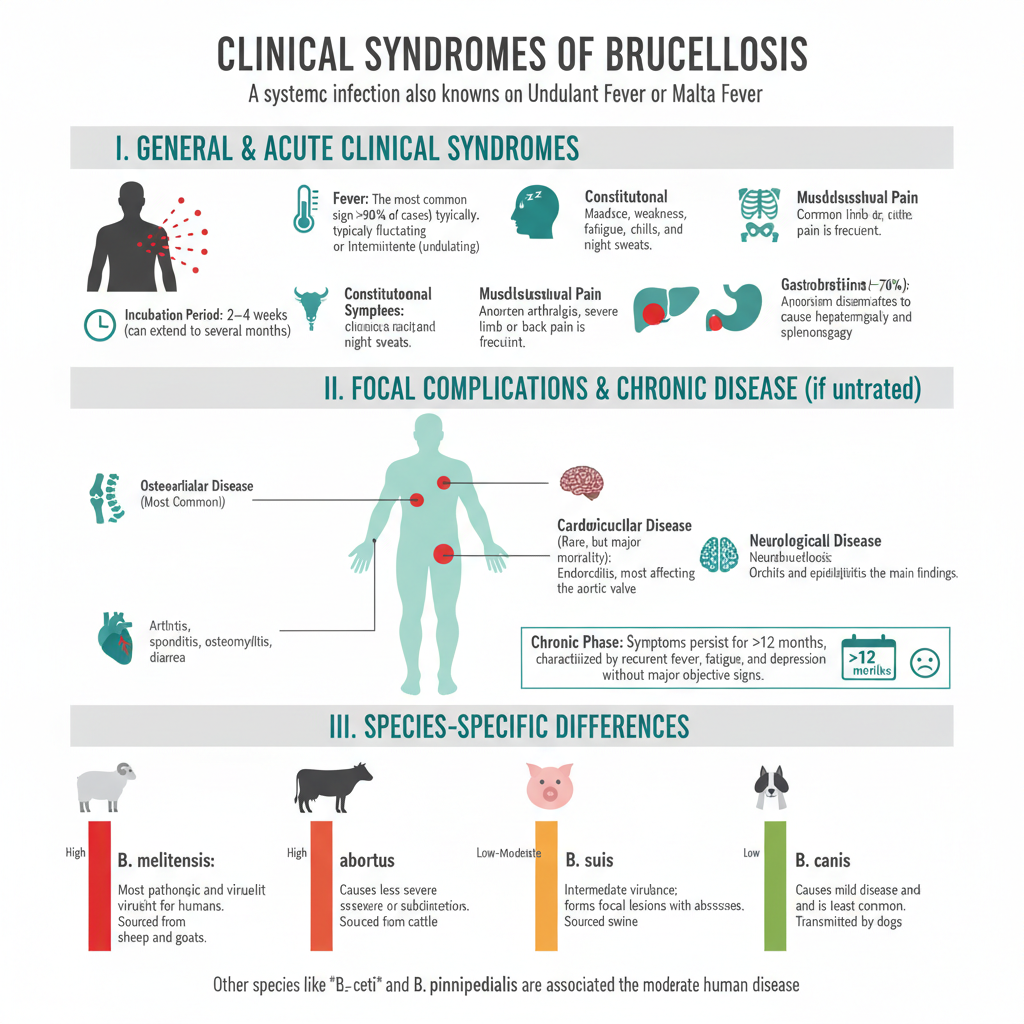
Clinical Syndromes of Brucellosis
General and Acute Clinical Syndromes
- It is a systemic infection in humans caused by different Brucella species.
- It is also referred to as undulant fever or Malta fever.
- The incubation period is usually 2–4 weeks but sometimes it may take several months.
- Common acute manifestations are–
- Fever – It is the most common sign and is usually fluctuating or intermittent (undulating). The cyclical fever is present in more than 90% cases.
- Constitutional symptoms – These are malaise, headache, weakness, fatigue, chills and night sweats.
- Musculoskeletal pain – Arthralgia is highly common and limb or back pain is often severe.
- Gastrointestinal and visceral signs – It is the process in which the organism disseminates to reticuloendothelial tissues. Hepatomegaly and splenomegaly can occur. Around 70% cases show anorexia, abdominal pain, vomiting or diarrhoea.
Focal Complications and Chronic Disease
- If the infection is untreated, it may progress into chronic phase.
- Focal syndromes involve different organ systems.
- Some of the major focal complications are–
- Osteoarticular disease – It is the most common focal form. Arthritis, spondylitis, osteomyelitis and sacroiliitis are seen.
- Cardiovascular disease – Endocarditis is rare (0.3–2%) but it is the major cause of mortality. Aortic valve is affected mostly.
- Neurological disease – Neurobrucellosis and meningitis may occur.
- Genitourinary disease – Orchitis and epididymitis are the main findings.
- Chronic phase – It is when symptoms persist for more than 12 months. It is characterized by recurrent fever, fatigue and depression without major objective signs.
Species-Specific Differences in Human Pathogenicity
- Some of the main features of species involved are–
- B. melitensis – It is the most pathogenic and virulent for humans. It is isolated mainly from sheep and goats.
- B. abortus – It is from cattle and usually causes less severe or subclinical infection. Infection by vaccine strain RB51 occur by accidental exposure or raw milk consumption.
- B. suis – It is associated with swine. It has intermediate virulence and forms focal lesions with abscess formation.
- B. canis – Transmitted by dogs. It causes mild disease and is least common.
- Other species – B. ceti, B. pinnipedialis, B. inopinata, and B. neotomae are associated with moderate human disease.
Reservoir, and Source of Brucellosis
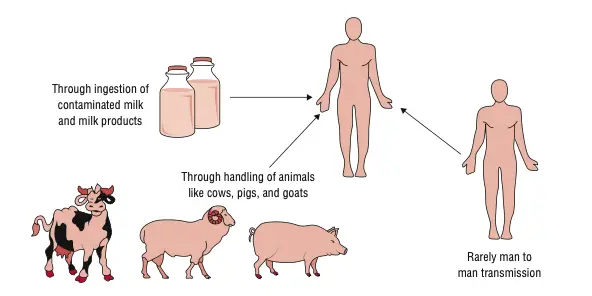
Reservoir of Brucellosis
- Livestock is the major reservoir of the organism.
- Cattle maintain B. abortus and this species is responsible for a continuous zoonotic cycle.
- Goats and sheep is the main host for B. melitensis which is the most pathogenic type.
- Pigs carry B. suis and these herds support long-term persistence of the organism.
- Dogs infected with B. canis act as domestic reservoirs, and this is seen in household transmission.
- Wild rodents like those carrying B. neotomae or B. microti is responsible for environmental circulation.
- Sheep infected by B. ovis create flock-level maintenance of infection.
- Marine mammals such as seals and whales harbor B. ceti and B. pinnipedialis. These are unusual aquatic reservoirs.
- Wildlife populations like bison, elk, deer and wild boar maintain B. abortus in free-ranging areas and allow back-spill to livestock.
Source of Human Infection
- The major source is unpasteurized milk and dairy products (milk, cheese) from infected herds.
- Raw or undercooked meat from livestock or wildlife can act as a source depending on preparation practices.
- Direct contact with infected tissues is important. These tissues include placenta, fetus or genital secretions.
- Ingestion of contaminated food or drink is a common transmission mode.
- Inhalation of aerosols occur in laboratories or abattoirs and this is considered a high-risk situation.
- Entry through skin abrasion or mucosal inoculation is another route.
- Other less common sources are conjunctival contact, blood transfusion and rare sexual transmission.
- Occupational groups such as farmers, veterinarians, slaughterhouse workers and laboratory staff are repeatedly exposed.
- Hunters and consumers of game meat may get infection when wildlife species carry Brucella.
- Wildlife dynamics show transmission by wild bison, elk and deer for B. abortus, while marine species transmit B. ceti through reproductive fluids or vertical pathways.
- Overall, human cases arise when ingestion, inhalation or direct contact occur with infected materials or products.
Brucellosis Transmission Process to Humans
- It is a zoonotic process where humans act as accidental hosts.
- The infectious dose is very low, usually between 10 to 100 organisms.
- After entry, the bacteria penetrate the epithelial surface and it is internalized by macrophages or dendritic cells.
- It is then disseminated in the bloodstream and reaches spleen, liver and lymph nodes.
1. Ingestion of Contaminated Products (Dietary Exposure)
- It is a major non-occupational route.
- Unpasteurized dairy – Intake of raw milk or fresh soft cheeses is the most common source.
- Contaminated meat – Eating raw or undercooked meat from livestock or feral swine can cause infection.
- Contaminated food or water – Animal discharges can contaminate feed and this is another route.
2. Direct Contact with Infected Animals or Tissues (Occupational Exposure)
- It is the primary mode for people working with animals.
- Handling infected materials – Contact with blood, urine, semen, or secretions from infected animals.
- Products of abortion/parturition – Placenta, aborted fetus or uterine fluids contain high organism load.
- Routes of entry – Entry occurs through cuts, abrasions or mucosal membrane contact (mouth or conjunctiva).
- High-risk occupations – Farmers, veterinarians, abattoir workers, and hunters handling carcasses.
3. Inhalation of Aerosols (Respiratory Exposure)
- Infectious aerosols can cause transmission.
- Contaminated dust or aerosols – These create infection in industrial or crowded environments.
- Laboratory exposure – It is the most common laboratory-associated bacterial infection.
- Aerosols generated during centrifugation or subculture increase the risk because of the low infectious dose.
4. Person-to-Person Transmission (Exceptional Routes)
- It is very rare and unusual.
- Vertical or neonatal transmission – Infection may occur by transplacental route or during delivery.
- Breastfeeding – It can transmit B. melitensis when the mother is bacteremic.
- Sexual transmission – Rare reports are present.
- Iatrogenic transmission – Blood transfusion or tissue transplantation may transfer infected cells.
Laboratory Diagnosis of Brucellosis
Laboratory confirmation is important because symptoms is usually non-specific.
Three main approaches are used which is culture, serological tests and nucleic acid amplification methods.
Work with live Brucella must be done under BSL-3 conditions because the organism is highly infectious.
1. Culture (Isolation of the Agent)
- Culture is considered as the definitive test because isolation gives direct evidence of infection.
- It helps in identifying species and biovar which is important for epidemiology.
Specimen collection
- Blood culture – It is the method of choice in early disease because bacteremia is continuous.
- Bone marrow culture – It is more sensitive than blood and is positive in most cases but the procedure is invasive.
- Other samples – CSF, joint fluid, urine, tissue biopsies and milk may also contain organisms.
Culture conditions
- Brucella is slow growing and may take up to 21 days for visible growth.
- Automated systems detect CO₂ production and reduce detection time.
- Biphasic medium like Castañeda helps in isolation from blood.
- Solid media include TSA and Brucella agar.
- Failure to grow on MacConkey agar is a characteristic feature.
- Incubation is done at around 35°C with 5–10% CO₂.
Presumptive identification
- Small faint Gram-negative coccobacilli is seen on Gram stain.
- Organisms are weakly acid-fast by Stamp staining.
- Oxidase, catalase and urease tests is positive.
- No sugar fermentation and no motility is observed.
2. Serological Tests
- Serology is the main approach when cultures are negative.
- Tests show exposure but do not identify the species exactly.
Diagnostic criteria
- A four-fold rise in antibody titre between acute and convalescent samples confirms infection.
- A titre of ≥1:160 by SAT or BMAT is considered presumptive.
Common assays
- SAT and BMAT – It is used for smooth species and a titre ≥1:160 is diagnostic with clinical illness.
- Rose Bengal Test – It is a rapid screening test.
- 2-ME test – It helps in detecting IgG by removing IgM activity.
- CFT – Used for confirmation because of high specificity.
- ELISA – Detects IgG, IgM or IgA and is useful for chronic cases.
- Coombs/Brucellacapt – Detects incomplete antibodies in relapse or chronic infection.
Drawbacks
- Cross-reaction occur with Yersinia enterocolitica O:9.
- Cannot detect infection with rough strains like B. canis or RB51.
- Chronic cases may show low or inconsistent agglutination titres.
3. Molecular Methods (NAATs)
- PCR and related tests offer rapid and highly sensitive detection.
- These methods are safer because no live organism handling is needed.
Targets and types
- PCR detecting genes such as BCSP31 or 16S rRNA.
- Multiplex PCR (Bruce-Ladder, AMOS) help in species differentiation.
- RPA assays is being developed for rapid species-specific detection.
Advantages and limitations
- It can detect DNA from blood, urine and paraffin-embedded tissues.
- Persistence of DNA after treatment is common, so positive PCR does not always indicate relapse.
4. Advanced and Adjunct Methods
- MALDI-TOF MS – Used for rapid species-level identification after inactivation.
- Brucellin skin test – Applied mainly in animals for herd screening.
- Phenotypic identification – Reference labs perform phage lysis and monospecific sera agglutination.
Treatment of Brucellosis
Treatment is difficult because the organism survive inside macrophages. It is the process that require drugs which can enter cells and act for long duration. Single-drug therapy is not recommended as relapse is high. Combination therapy for at least six weeks is commonly used.
Standard Treatment for Uncomplicated Cases
- Doxycycline + Rifampin (DR regimen)
- Doxycycline is given in divided doses.
- Rifampin is added as second drug.
- It is fully oral but may cause nausea and loss of appetite.
- Relapse may occur more than other dual regimens.
- Doxycycline + Streptomycin (SD regimen)
- Streptomycin injection is used with doxycycline.
- Failure and relapse rates is lower than DR regimen.
- Alternative dual therapy
- Doxycycline + Gentamicin – It shows moderate failure and relapse rates.
- TMP-SMX + Doxycycline – Used when tetracyclines cannot be given.
- Fluoroquinolone + Rifampin – It is used, but relapse is variable.
Special Considerations (RB51 Vaccine Strain)
- RB51 strain is naturally rifampin-resistant.
- Rifampin should not be used for treatment or PEP for this strain.
- PEP – Doxycycline plus another suitable drug like TMP-SMX for 21 days.
- Confirmed RB51 infection – TMP-SMX may replace rifampin in combination therapy.
Management of Complicated and Severe Cases
- Complicated disease require prolonged and aggressive therapy.
- Triple-drug therapy is often used for at least three months.
- Doxycycline + Rifampin + an aminoglycoside (Streptomycin or Gentamicin).
- It is considered more effective for preventing relapse.
- Endocarditis
- It is the most serious complication.
- Medical plus surgical management is required.
- Triple antibiotics for six weeks pre-operative, then extended course after surgery.
Treatment in Specific Groups
- Children
- Doxycycline is generally avoided.
- TMP-SMX + Rifampin is commonly used.
- Streptomycin for two weeks may reduce relapses.
- Pregnancy
- Rifampin for six weeks is widely recommended.
- Rifampin + TMP-SMX may also be used.
- Risk of spontaneous abortion requires urgent treatment.
Novel Therapeutic Approaches
- Research is focusing on drugs that disrupt intracellular survival.
- ER-interaction targeting
- The organism induce UPR in host cells through TcpB.
- Drugs that inhibit UPR may reduce replication.
- Herbal compounds
- Ginseng fractions like RGSF-A show effect on intracellular trafficking.
- Components such as ginsenoside Rg3 help in fusion with lysosomal markers (LAMP-1).
- Plants like Teucrium polium, Eucalyptus, garlic and barberry roots are also being studied.
Prevention and Control of Brucellosis
Control in Domestic Animals
- Prevention depend mainly on removing infection from animal reservoirs.
- Vaccination is widely used in cattle, sheep and goats to reduce abortion and infection.
- Strain 19 and RB51 vaccine is used for B. abortus control in cattle.
- Rev.1 strain is the vaccine for B. melitensis in sheep and goats.
- Vaccine interference with serological tests is seen with S19, so it is given only to young calves.
- RB51 does not induce smooth-LPS antibodies, so it can be used at any age.
- No suitable vaccine is available for B. suis in swine.
- Test-and-slaughter program remove infected animals after diagnostic testing.
- Surveillance with proper laboratory identification helps to track infected herds.
- Wildlife reservoirs like bison, elk and feral swine create difficulty in eradication.
Public Health and Food Safety Measures
- Pasteurization of milk and milk products is the most effective measure.
- Unpasteurized milk and fresh soft cheeses are a major risk in endemic regions.
- Travelers and immigrants should avoid raw dairy products.
- Hunters must avoid handling raw pork from feral swine without protection.
- Dogs should not be allowed to eat or play with carcasses.
Occupational and Laboratory Safety
- Farmers, veterinarians, abattoir workers and laboratory staff need protective clothing.
- Gloves, face masks, boots and impermeable aprons reduce exposure.
- Contact with placenta, fetal fluids and uterine discharges is high-risk.
- Laboratory work with Brucella must be done under BSL-3 conditions.
- Class II biological safety cabinet is used to prevent aerosol formation.
- PPE must be worn, and all procedures should minimize spills and splashes.
- Post-exposure prophylaxis is recommended for high-risk incidents.
- RB51 exposure cannot be monitored with routine serology; symptoms must be observed.
Prevention of Person-to-Person Transmission
- Person-to-person transmission is very rare but precaution is needed.
- Pregnant women must avoid unpasteurized dairy and contact with infected animals.
- Bacteremia during pregnancy may cause abortion or transmit infection to the infant.
- Neonatal infection may occur during delivery or through breastfeeding.
- Alton GG, Forsyth JRL. Brucella. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 28. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8572/
- Carvalho TPD,Silva LAD, Castanheira TLL,Souza TDD, Paixão TAD, Lazaro-Anton L, Tsolis RM, Santos RL,2023.Cell and Tissue Tropism of Brucella spp.. Infect Immun91:e00062-23.https://doi.org/10.1128/iai.00062-23
- https://emedicine.medscape.com/article/213430-overview
- https://www.woah.org/en/disease/brucellosis/
- https://www.webmd.com/a-to-z-guides/brucellosis-symptoms-treatment
- https://www.sciencedirect.com/topics/immunology-and-microbiology/brucella
- https://medlineplus.gov/ency/article/000597.htm
- https://www.msdmanuals.com/professional/infectious-diseases/gram-negative-bacilli/brucellosis
- https://www.cdc.gov/brucellosis/pdf/brucellosi-reference-guide.pdf
- https://www.mayoclinic.org/diseases-conditions/brucellosis/symptoms-causes/syc-20351738
- https://my.clevelandclinic.org/health/diseases/17886-brucellosis
- https://www.cdc.gov/brucellosis/about/index.html
- https://www.who.int/news-room/fact-sheets/detail/brucellosis
- https://emedicine.medscape.com/article/213430-overview
- https://en.wikipedia.org/wiki/Brucella