What is Bordetella pertussis?
- Bordetella pertussis is a Gram-negative, aerobic, encapsulated coccobacillus bacterium that serves as the primary cause of pertussis, commonly known as whooping cough. It belongs to the genus Bordetella, which includes several other species like B. bronchiseptica and B. parapertussis. The bacterium is highly pathogenic due to a range of virulence factors, such as pertussis toxin, adenylate cyclase toxin, filamentous hemagglutinin, pertactin, fimbriae, and tracheal cytotoxin. These factors enable the bacterium to adhere to and damage respiratory tissues, contributing to the characteristic symptoms of whooping cough, including severe coughing fits.
- The transmission of B. pertussis occurs primarily through airborne droplets expelled when an infected person coughs or sneezes. The incubation period typically ranges from 7 to 10 days, though it can extend from 6 to 20 days in some cases. Notably, humans are the sole known reservoir for B. pertussis, and the bacterium does not survive outside the human host. This specificity to humans is one reason for its limited survival and the eventual reduction in genome size compared to its close relatives, such as B. bronchiseptica. Sequencing of the B. pertussis genome, completed in 2003, revealed that it consists of 4,086,186 base pairs, a notably smaller genome than that of B. bronchiseptica, reflecting its adaptation to a single host.
- Interestingly, while B. pertussis is typically considered nonmotile, it can express flagellum-like structures under certain conditions, a characteristic it shares with its relatives, such as B. bronchiseptica. This motility is not utilized for movement but may serve other purposes related to host interaction.
- The genus Bordetella includes several other species besides B. pertussis, such as B. parapertussis, which causes a disease similar to whooping cough in humans, and B. bronchiseptica, which infects a range of mammal species, including humans. B. bronchiseptica is responsible for a variety of respiratory illnesses in mammals. The relationship between these species is phylogenetically close, and they share several key genetic traits that allow them to cause similar respiratory diseases across different host species.
- The history of pertussis dates back to the 16th century, with the disease first being described by French physician Guillaume de Baillou after a major epidemic in 1578. However, earlier references to similar diseases may have appeared in Korean medical texts. In 1906, scientists Jules Bordet and Octave Gengou isolated B. pertussis as the causative agent of the disease, solidifying its role in pertussis infections. It is believed that the genus Bordetella evolved from soil-dwelling ancestors, with genetic evidence suggesting that its transition to infect humans was facilitated by increased human interaction with soil during agricultural development. This shift enabled Bordetella species like B. pertussis to adapt to human hosts while still retaining the ability to thrive in soil environments.
- Recent advancements in genomic analysis have revealed that B. pertussis strains show variations in their genetic makeup, especially in the ptxP region of the genome. The ptxP3 allele, a relatively recent mutation, has become more prevalent in many parts of the world, including developed countries like the United States. This allele leads to increased expression of pertussis toxin, making the pathogen more virulent and causing more severe cases of the disease. Studies have shown that the ptxP3 strain is now the dominant form in many regions, and this shift has contributed to a rise in pertussis incidence in both developed and developing countries. The increase in intragenomic recombination and the loss of certain genes have also been linked to the rise in B. pertussis virulence, further underscoring the bacterium’s adaptive capacity.
- Thus, B. pertussis continues to be a significant pathogen, with evolving strains influencing the course and severity of pertussis outbreaks globally. Its unique genetic adaptations, along with its reliance on human hosts, make it a challenging bacterium to control and prevent.
Scientific classification of Bordetella pertussis
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Betaproteobacteria |
| Order: | Burkholderiales |
| Family: | Alcaligenaceae |
| Genus: | Bordetella |
| Species: | B. pertussis |
Geographical Distribution and Habitat of B. pertussis
Bordetella pertussis, the causative agent of whooping cough, is a major concern worldwide. The disease it causes is predominantly seen in children, and its spread is influenced by both geographical factors and the specific habitats where the bacteria thrive.
- Geographical Distribution:
- Whooping cough is endemic across the globe, affecting millions of people each year.
- The disease results in over 600,000 cases annually, with 51 million cases and 600,000 deaths reported globally, especially in regions where vaccination is not consistently practiced.
- Countries with lower vaccination rates face a higher burden of pertussis, leading to significant childhood mortality.
- Habitat:
- B. pertussis specifically targets the cilia of the respiratory epithelium in mammals, primarily in infected children.
- The bacteria colonize these ciliated cells, enabling them to evade the immune system and perpetuate the infection within the respiratory tract.
Morphology of Bordetella pertussis
Bordetella pertussis is a small, Gram-negative bacterium responsible for causing pertussis, also known as whooping cough. The bacterium exhibits distinctive structural features that contribute to its identification and behavior. Here are the key characteristics of B. pertussis morphology:
- Shape and Size: B. pertussis is an extremely small, ovoid coccobacillus, measuring between 0.2 and 0.5 micrometers in diameter. These tiny cells are generally single or occur in pairs.
- Pleomorphism: The bacterium demonstrates pleomorphism, meaning its shape can vary under different conditions. This variation is a hallmark of B. pertussis and may affect its appearance during microscopic examination.
- Gram Staining: B. pertussis is Gram-negative, which means it does not retain the crystal violet stain used in Gram staining but takes up the counterstain, resulting in a pink appearance under the microscope.
- Nonmotile and Nonsporing: This bacterium does not possess flagella for motility and does not form spores, both of which are important to consider when understanding its behavior in the host and environment.
- Capsule and Fimbriae: Freshly isolated strains of B. pertussis have a poorly defined capsule. The capsule’s presence is a part of the bacterium’s ability to evade host immune defenses. Additionally, fimbriae (hair-like appendages) are present and play a role in adhesion to host tissues.
- Staining Characteristics: When stained with Toluidine blue, B. pertussis shows bipolar metachromatic granules, which appear as colored spots at the ends of the cells. This staining method helps distinguish B. pertussis from other bacteria.
- Cultural Appearance: On solid culture media, the bacteria often appear in loose clumps with clear spaces in between, giving a characteristic thumbprint appearance. This feature is especially noticeable in Gram-stained culture smears.
Culture and Biochemical Reactions of Bordetella pertussis
Bordetella pertussis is a highly specialized bacterium that exhibits specific growth requirements and biochemical characteristics. These features play a crucial role in its identification and understanding how it interacts with its environment.
Culture Characteristics
- Strict Aerobe: B. pertussis requires oxygen for growth, making it a strict aerobe. It thrives best at an optimum temperature of 35ºC.
- Nutritionally Fastidious: This bacterium is particularly demanding in terms of nutritional needs. It does not grow on common laboratory media like blood agar or nutrient agar.
- Slow Growth on Blood Agar: When grown on blood agar, B. pertussis forms pinpoint colonies that require 3–6 days to appear. This slow growth is a distinctive feature of the bacterium.
- Rich Media for Cultivation: The bacterium grows best on rich media that is supplemented with various nutrients. These include charcoal, starch, blood, albumin, and essential growth factors like nicotinamide. Nicotinamide is crucial for the bacterium’s growth.
- Blood and Albumin in Medium: Blood or albumin in the growth medium doesn’t serve as a direct nutrient source. Instead, it neutralizes toxic substances like fatty acids present in the agar, which could inhibit bacterial growth.
- Bordet–Gengou Agar: For primary isolation, B. pertussis is often cultured on Bordet–Gengou agar, which contains 15–20% blood. After 48–72 hours of incubation, the bacterium forms small, smooth, grayish-white colonies that appear refractile and resemble bisected pearls or mercury drops. A hazy zone of hemolysis is often visible around the colonies. The overall growth on this medium may take on an aluminum paint appearance when the bacteria grow in large clusters.
- Charcoal Agar: Charcoal agar with 10% blood is another medium used for the primary isolation of B. pertussis. This medium also supports growth but is less commonly used than Bordet–Gengou agar.
- MacConkey Medium: B. pertussis does not grow on MacConkey medium, although other species in the genus Bordetella can.
Biochemical Reactions
- Oxidase and Catalase Positive: B. pertussis tests oxidase positive and catalase positive, indicating it has enzymes that play roles in oxidative reactions and breaking down hydrogen peroxide.
- Biochemically Inert: Despite these positive reactions, B. pertussis is largely biochemically inert, meaning it does not exhibit many of the common biochemical reactions observed in other bacteria.
- Non-fermenting: This bacterium does not ferment carbohydrates, which is a typical feature of aerobic organisms that rely on other metabolic pathways.
- Lack of Other Biochemical Activities: B. pertussis does not produce indole, reduce nitrate, split urea, or utilize citrate as carbon sources. These reactions are often used to differentiate B. pertussis from other bacteria in the genus Bordetella and from other species entirely.
Cell Wall Components and Antigenic Properties of Bordetella pertussis
Bordetella pertussis has several distinctive features in its cell wall structure and antigenic properties, which are crucial for its identification, classification, and understanding of its pathogenic mechanisms.
Cell Wall Components
- Gram-negative Structure: B. pertussis is a Gram-negative bacterium, meaning it has a characteristic outer membrane that contains lipopolysaccharides (LPS).
- Unusual LPS: The LPS in B. pertussis is heterogeneous and includes two distinct types: lipid A and lipid X. These molecules are significant in immune response.
- Complement Activation: Both lipid A and lipid X are capable of activating the alternative pathway of complement. This process plays a role in the immune system’s response to infection.
- Cytokine Release: These LPS components can also stimulate the release of cytokines, which are key signaling molecules involved in the immune system’s defense.
- Role in Pathogenesis: The exact role of B. pertussis‘s unusual LPS in the development of whooping cough is still not fully understood, but it clearly contributes to immune system interaction.
Antigenic Properties
- Somatic O Antigen:
- The O antigen is genus-specific and found in most strains of Bordetella.
- It is protein-based and heat-stable, making it resilient to heat.
- This antigen is of a single antigenic type, which is common across most B. pertussis strains.
- Capsular K Antigen:
- The K antigen is strain-specific and can vary between different isolates.
- It is heat-labile, meaning it is more sensitive to heat than the O antigen.
- These K antigens are crucial for differentiating various strains of B. pertussis, especially in epidemiological studies where tracking different strains is important.
- Agglutinogens and Serotyping:
- A total of 14 agglutinating factors (also called agglutinogens) have been identified through the agglutinin absorption test. These factors help in identifying and grouping different strains.
- Based on these agglutinogens, B. pertussis strains are classified into various types, which helps in understanding the diversity of the pathogen and its behavior.
- Factor 7 is present in all B. pertussis strains, as well as in strains of B. parapertussis and B. bronchiseptica.
Virulence Factors of Bordetella pertussis
Bordetella pertussis relies on several virulence factors to colonize the respiratory tract and evade the host’s immune system. These factors play critical roles in adhesion, immune evasion, and damage to host tissues, all of which contribute to the severity of whooping cough.
Key Virulence Factors
- Filamentous Hemagglutinin (FHA):
- FHA is the most crucial virulence factor of B. pertussis.
- It is a 220 kDa protein that forms filamentous structures on the bacterial surface.
- FHA binds to galactose residues on a glycolipid called sulfatide, found on ciliated epithelial cells in the respiratory tract.
- It also binds to CR3, a receptor on the surface of polymorphonuclear leukocytes, facilitating bacterial survival inside the leukocytes.
- This interaction protects B. pertussis from humoral antibodies targeting FHA.
- Mutations in the gene for FHA can reduce bacterial colonization.
- The gene for FHA has been cloned, opening the door for potential vaccine development.
- Pertussis Toxin:
- Pertussis toxin is a 105 kDa protein composed of two subunits: A (enzymatically active) and B (binding subunit).
- The B subunit facilitates binding to the epithelial cells of the trachea, and the A subunit enters the host cells, disrupting normal cellular function.
- The S2 subunit of the B unit binds to a glycolipid on ciliated epithelium, while the S3 subunit binds to ganglioside receptors on phagocytic cells.
- Once inside, the A subunit inhibits adenylate cyclase activity by transferring an ADP-ribosyl group to G1, disrupting cell signaling and killing phagocytes.
- The gene for pertussis toxin has been cloned, and the toxin is toxoided for use in vaccines.
- Invasive Adenylate Cyclase (Hemolysin):
- This 45 kDa protein has dual roles as both an enzyme and an adhesin.
- The adenylate cyclase activity increases cyclic AMP (cAMP) levels, disrupting cellular signaling and immune responses.
- It also mediates bacterial attachment to the host cell surface, aiding in colonization.
- This protein was initially identified as a hemolysin, as it causes lysis of blood cells. The lysis results in a characteristic zone of hemolysis on blood agar plates.
- Lethal Toxin:
- Known previously as dermonecrotic toxin, this 102 kDa protein causes inflammation and necrosis at the site of bacterial adhesion.
- It induces lethal necrosis in experimental models, particularly at high doses.
- While its exact role in disease pathogenesis remains unclear, it contributes to the local damage seen in infection.
- Tracheal Cytotoxin:
- Unlike other toxins, tracheal cytotoxin is not protein-based; it consists of a peptidoglycan fragment.
- It is toxic to ciliated respiratory cells, leading to cell death and inhibition of ciliary movement.
- The toxin stimulates the release of interleukin-1 (IL-1), contributing to fever and inflammation.
- It specifically inhibits DNA synthesis in the respiratory cells, preventing their regeneration after damage.
Other Factors
- Adhesins and Pertactin:
- B. pertussis also produces other adhesins and pertactin, but their precise role in the infection process and adherence remains unclear.
Pathogenesis of Pertussis
The pathogenesis of pertussis, caused by Bordetella pertussis, begins with bacterial attachment to the ciliated epithelial cells in the respiratory tract. This attachment is critical for the development of the disease and is facilitated by bacterial adhesions like FHA (filamentous hemagglutinin) and pertussis toxin. Once attached, the bacteria begin to multiply, producing several toxins that damage the respiratory system and lead to the characteristic symptoms of whooping cough.
- Initial Attachment and Colonization:
- The bacteria initially latch onto ciliated epithelial cells through FHA and pertussis toxin.
- Pertussis toxin plays a double role in both colonization and the toxemic stage of the disease.
- The S2 subunit of pertussis toxin specifically binds to a glycolipid receptor on the ciliated cells, aiding in further bacterial adherence.
- Toxin Production and Effects:
- Once attached, the bacteria produce pertussis toxin, which plays a major role in pathogenesis.
- The toxin increases cyclic AMP (cAMP) levels in the host cells, leading to excessive respiratory secretions and mucus production, which characterizes the paroxysmal stage of whooping cough.
- The S1 subunit of pertussis toxin directly causes inflammation in the respiratory tract, exacerbating the condition.
- Adenylate Cyclase Toxin:
- In addition to pertussis toxin, Bordetella pertussis also produces adenylate cyclase toxin.
- This toxin inhibits important immune responses, including leukocyte chemotaxis (movement of white blood cells to infection sites), phagocytosis (engulfing of bacteria by immune cells), and the killing of the bacteria itself.
- This allows the bacteria to evade the immune system and persist in the respiratory tract.
- Bacterial Inflammation and Damage:
- The combination of FHA, pertussis toxin, and adenylate cyclase toxin creates a cycle of inflammation and immune suppression, leading to the characteristic coughing fits and respiratory distress associated with pertussis.
Toxins of B. pertussis Infection
The pathogenesis of Bordetella pertussis involves a variety of toxins that play crucial roles in establishing infection and evading the host immune system. These toxins disrupt normal immune responses, contributing to the severity of pertussis.
- Pertussis Toxin (PT):
- PT is one of the primary virulence factors identified in B. pertussis.
- Known for inducing lymphocytosis, PT has been extensively studied and is linked to the immune system’s disruption during infection.
- PT is an AB5-type toxin consisting of five binding subunits (B) and a catalytic subunit (A).
- Once PT binds to host cells, it enters through receptor-mediated endocytosis, traveling to the Golgi apparatus and endoplasmic reticulum (ER).
- The A subunit modifies G proteins by ADP-ribosylation, leading to increased cyclic AMP (cAMP) levels inside cells, disrupting various signaling pathways, including immune responses.
- PT also inhibits the migration of immune cells such as neutrophils, monocytes, and lymphocytes, affecting the body’s ability to fight off infection.
- The toxin is linked to increased bacterial burden and suppression of early inflammatory responses.
- It is associated with the extreme lymphocytosis observed in pertussis patients, and antibodies against PT are protective against severe disease.
- Adenylate Cyclase Toxin (ACT):
- ACT is another significant toxin produced by B. pertussis and other Bordetella species.
- ACT is part of the RTX toxin family and operates by producing cAMP, similar to PT, but with a different mechanism.
- This toxin binds to complement receptor 3 (CR3) on neutrophils, macrophages, and dendritic cells, disrupting their function.
- ACT inhibits phagocytosis, oxidative burst, and T-cell activation.
- In animal models, ACT-deficient bacteria are cleared faster than those producing ACT, suggesting its importance in immune evasion.
- Type III Secretion System (T3SS):
- The T3SS is involved in injecting bacterial effector proteins into host cells, causing cell death and immune modulation.
- The BteA effector protein is critical for cytotoxicity, but its exact mechanism remains unclear.
- T3SS activity is regulated by several factors, including iron starvation and BtrS, and it is implicated in the persistence of the bacteria in the lower respiratory tract.
- Tracheal Cytotoxin (TCT):
- TCT is a peptidoglycan fragment released by B. pertussis during cell wall remodeling.
- TCT promotes pro-inflammatory cytokine production and the destruction of ciliated epithelial cells in the respiratory tract.
- The toxin acts via NOD1, a host receptor that recognizes bacterial peptidoglycan and triggers inflammation.
- Although TCT contributes to airway damage and may be linked to the characteristic cough of pertussis, its role in human infection requires further study.
- Dermonecrotic Toxin (DNT):
- DNT causes necrotic lesions when injected into animals and plays a role in the pathology of respiratory infections in certain animal models.
- This toxin has transglutaminase activity and can activate Rho GTPases, which are involved in cell signaling and survival.
- While DNT does not seem to be secreted from bacterial cells in culture, it may function within the bacterial cytoplasm during infection, aiding survival and pathogenesis.
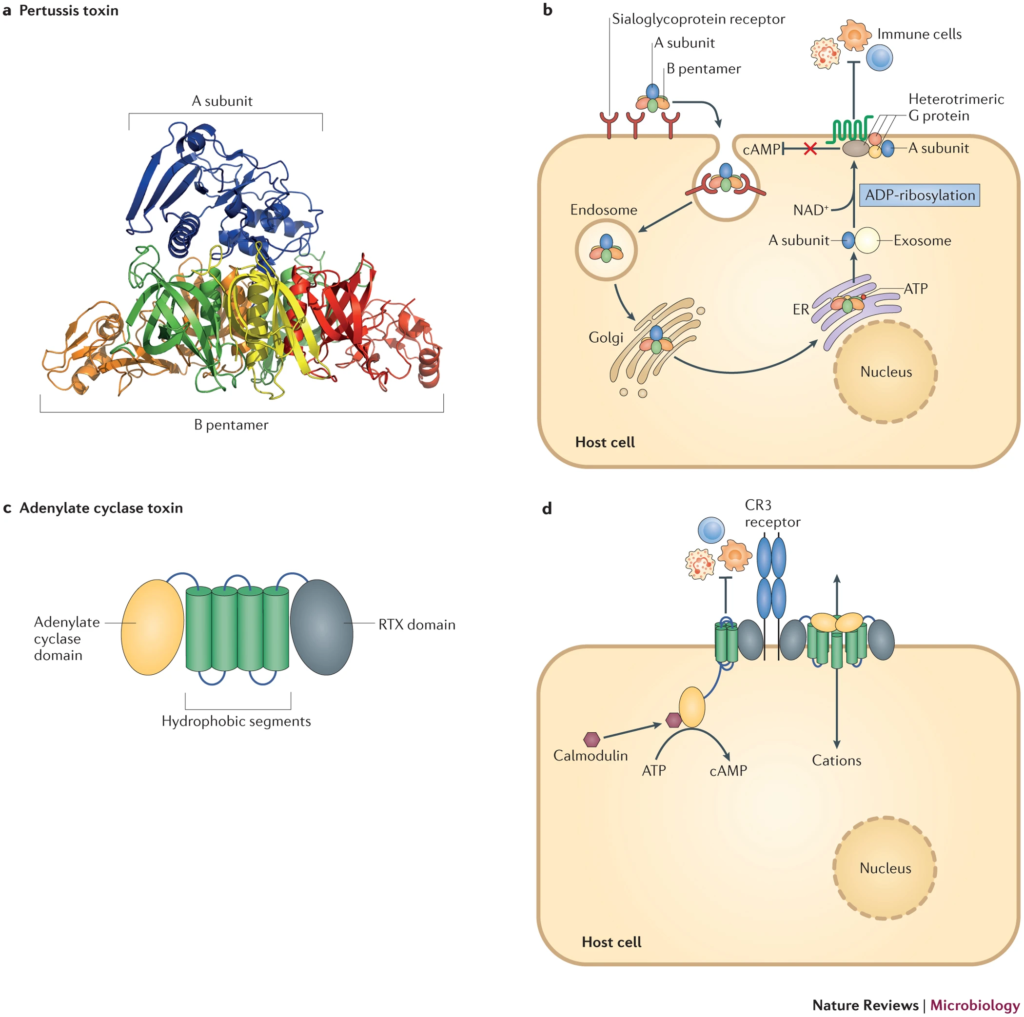
Toxin-Mediated Virulence Mechanism of Bordetella spp.
Bordetella species, the culprits behind respiratory infections like whooping cough, owe their harmful effects to a variety of toxins. These toxins disrupt immune responses and facilitate bacterial persistence in the host. Here’s a breakdown of how Bordetella spp. use toxins to outsmart the immune system and thrive in the host environment:
- Pertussis Toxin (PT)
- Structure: PT is an AB5-type toxin made up of one catalytic A subunit and five membrane-binding B subunits.
- Entry into Host Cells: PT binds to sialoglycoproteins on host cells, triggering endocytosis.
- Intracellular Trafficking: Once inside, PT follows a retrograde pathway, moving from the Golgi apparatus to the endoplasmic reticulum (ER).
- Mechanism of Action: The A subunit is released into the cytoplasm, where it modifies G proteins by ADP-ribosylation. This modification inhibits G protein-regulated pathways, particularly those controlling cyclic AMP (cAMP) production.
- Resulting Effects: Elevated cAMP levels disrupt immune function by suppressing cytokine production, inhibiting immune cell migration, and preventing early inflammatory responses. This gives Bordetella a head start in establishing infection.
- Adenylate Cyclase Toxin (ACT)
- Structure: ACT contains two domains: an adenylate cyclase enzyme domain (calmodulin-dependent) and an RTX domain, which helps the toxin bind to host cells.
- Pore Formation: The RTX domain creates cation-selective pores in the host cell membrane, allowing for the influx of ions.
- Adenylate Cyclase Activity: Once inside the cell, the calmodulin-dependent cyclase domain boosts cAMP production.
- Combined Effects: These actions result in multiple immune disruptions:
- Inhibition of Phagocytosis: By blocking complement-dependent phagocytosis, ACT ensures that immune cells can’t effectively clear the bacteria.
- Cytokine Modulation: ACT induces anti-inflammatory cytokines while suppressing pro-inflammatory responses, further hindering the immune response.
- Immune Cell Recruitment: By disrupting signaling pathways, ACT prevents immune cells from being recruited to the site of infection, allowing the bacteria to persist longer in the host.
- Type III Secretion System (T3SS)
- Role in Cytotoxicity: The T3SS, studied most extensively in B. bronchiseptica, is involved in injecting effector proteins directly into host cells. These proteins can trigger necrosis and contribute to immune evasion.
- Regulation and Activation: The secretion of these effectors is tightly controlled by a regulatory cascade, ensuring that they are released only when needed for pathogenesis.
- Functionality in B. pertussis: Although less studied in B. pertussis, recent evidence shows that T3SS may play a role in modulating inflammation and promoting bacterial persistence in the lungs. Mutants lacking T3SS activity are cleared faster from animal models, suggesting it aids in evading early immune responses.
- Tracheal Cytotoxin (TCT)
- Source and Action: TCT is a peptidoglycan fragment produced by Bordetella during cell wall remodeling. It is released into the extracellular environment, where it triggers inflammatory responses.
- Host Recognition: TCT is detected by host pattern recognition receptors like NOD1, which stimulates the release of pro-inflammatory cytokines. However, human NOD1 is less efficient at detecting TCT compared to mouse NOD1.
- Effect on Host: TCT causes ciliated cells in the trachea to be destroyed, contributing to respiratory symptoms like coughing. While its exact role in human pathogenesis remains unclear, its ability to drive inflammation is well-documented in animal models.
- Dermonecrotic Toxin (DNT)
- Action: DNT induces necrosis when injected into subcutaneous tissues, forming lesions in mice.
- Mechanism: DNT activates Rho GTPases, which influence cell processes like cytoskeletal rearrangements.
- Effect on Host: DNT’s direct impact on host cells, particularly its ability to inhibit osteogenesis, may help Bordetella establish an infection niche. However, DNT is not secreted from bacterial cells during in vitro growth, indicating it might function intracellularly during infection.
Each of these toxins has evolved to impair various aspects of the immune system, whether by inhibiting immune cell functions, altering inflammatory responses, or promoting bacterial survival. Through a complex interplay of these factors, Bordetella spp. successfully evade immune defenses and establish persistent infections.
Clinical Syndromes of Pertussis Infection
Pertussis, commonly known as whooping cough, is caused by Bordetella pertussis and is one of the most prevalent vaccine-preventable diseases in children. It unfolds in three main stages: catarrhal, paroxysmal, and convalescent. Each phase is distinct in its symptoms and duration, and understanding them is crucial for diagnosis and management.
- Catarrhal Stage:
- This is the initial phase of the infection, resembling a typical upper respiratory tract infection.
- Symptoms include runny nose, nasal congestion, sneezing, malaise, and occasional cough.
- A low-grade fever may also occur.
- The catarrhal stage lasts for about 1-2 weeks.
- High infectiousness is noted here due to the large number of bacteria present.
- Paroxysmal Stage:
- The hallmark of this phase is the development of paroxysms (repetitive bouts of coughing), followed by the characteristic inspiratory whoop.
- These paroxysms occur more frequently at night, with an average of 15 attacks in a 24-hour period.
- The attacks are intense, often ending in vomiting and exhaustion.
- During this stage, the respiratory tract produces excessive mucus, leading to airway obstruction.
- The duration of this stage varies, typically lasting from 1–6 weeks, but it can extend up to 10 weeks.
- Convalescent Stage:
- This is the recovery phase, which can last weeks to months.
- The chronic cough persists but becomes less paroxysmal.
- Complications during this stage are frequent and may include:
- Subconjunctival hemorrhage (bleeding under the eye)
- Respiratory distress
- Secondary bacterial pneumonia
- Neurological complications, such as convulsions, which can lead to long-term effects like epilepsy, paralysis, blindness, and deafness.
Reservoir, Source, and Transmission of B. pertussis Infection
Whooping cough, caused by Bordetella pertussis, is one of the most contagious diseases, and understanding its transmission is key to controlling its spread.
- Reservoir:
- Humans are the only known reservoir of B. pertussis.
- The bacteria do not persist in animals or the environment.
- Source of Infection:
- The primary source of infection is an infected individual, particularly children.
- Infected children, especially during the catarrhal stage, shed large amounts of bacteria and are the most infectious.
- Transmission:
- The infection spreads mainly through aerosolized droplets, which are expelled when an infected person coughs.
- Airborne transmission occurs when these droplets are inhaled by individuals nearby.
- Transmission can also occur through contact with contaminated surfaces that have been exposed to respiratory secretions or droplets from an infected person.
- Secondary attack rates are highest among unimmunized household contacts, ranging from 75–100%.
- At-Risk Populations:
- While whooping cough was once primarily a disease of young children (under 5 years), it is now also seen in older children and adults.
- Adults and older children contribute to about 25% of whooping cough cases.
- This shift is largely due to waning immunity over time, even in individuals who were vaccinated.
Laboratory Diagnosis of B. pertussis Infection
Diagnosing Bordetella pertussis infection involves various laboratory techniques, each with its own strengths and limitations. Accurate identification helps confirm infection and guides treatment.
- Serology:
- Detects agglutinating antibodies in the patient’s serum, offering high sensitivity and specificity.
- Useful in measuring the immune response and determining virulence.
- Best performed during the catarrhal phase of the illness.
- Less effective in infants due to delayed results, which often indicate that the disease has progressed too far.
- The use of ELISA kits has been developed to streamline the process.
- Microbiological Culture:
- Known for high specificity, but it has limited sensitivity.
- Ideal for monitoring antimicrobial resistance.
- Specimen handling, immunization status, and age can affect results.
- Culturing B. pertussis is difficult, and high bacterial loads are required for a positive result.
- The best time to isolate the pathogen is during the catarrhal or early paroxysmal stage of infection.
- The plates are incubated at 36°C under high humidity for 7–10 days before obtaining results.
- Classical PCR Assay:
- PCR is often the test of choice due to its quick results and high sensitivity.
- Commonly used primers target IS481 and IS1001 transposable elements in the bacterial DNA.
- Works well on infants and patients in the catarrhal phase.
- Can detect the pathogen in vaccinated individuals and those with atypical presentations.
- However, differentiation between B. pertussis and other Bordetella species (like B. bronchiseptica and B. holmesii) can be tricky due to similar genetic expressions.
- Singleplex PCR can help pinpoint the ptxS1 gene for better specificity.
- Direct Fluorescent Antibody (DFA) Testing:
- DFA provides direct results for detecting B. pertussis but has poor sensitivity and specificity.
- It involves staining nasopharyngeal secretions with fluorescent-modified antibodies that bind to B. pertussis or B. parapertussis.
- False positives are common, especially with polyclonal antibodies.
- Enzyme-Linked Immunosorbent Assay (ELISA):
- ELISA kits detect filamentous hemagglutinin (FHA) and anti-pertussis toxin antibodies (IgG, IgA, or IgM).
- Some kits combine antigens for increased sensitivity, though this may complicate result interpretation.
- It’s challenging to discern which specific antibody was detected without further testing.
Treatment of B. pertussis Infection
Treating Bordetella pertussis infection involves using antibiotics to target the bacteria and supportive care to manage symptoms.
- Antibiotics:
- Erythromycin is the preferred antibiotic and is effective in eradicating the bacteria from the respiratory tract.
- Other antibiotics like tetracycline, chloramphenicol, and ampicillin are also effective, although erythromycin remains the drug of choice.
- Antibiotics reduce the duration of infectivity, making it crucial to start treatment early in the infection.
- Supportive Care:
- While antibiotics address the bacterial infection, the main aspect of treatment is good nursing care.
- The supportive care focuses on managing symptoms and ensuring the comfort of the patient, especially during the more severe stages of the illness.
Prevention and Control of B. pertussis Infection
The best way to prevent the spread of Bordetella pertussis is through vaccination, along with effective control measures.
- Vaccination:
- Vaccination plays a key role in preventing whooping cough, especially in infants and young children.
- Pertussis vaccines are administered primarily to children under the age of 7 to protect against infection.
- There are two main types of pertussis vaccines:
- Whole-cell inactivated vaccine
- Acellular vaccine
- Vaccine Effectiveness:
- These vaccines have shown to be highly effective in preventing severe outcomes of pertussis.
- Though vaccination significantly reduces infection rates, its effectiveness may diminish over time, especially in older populations.
- Control Measures:
- Since B. pertussis is highly contagious, isolating infected individuals, especially during the contagious stages, helps limit its spread.
- Good hygiene practices, including regular hand washing and covering the mouth when coughing, also aid in controlling the transmission.
- Finger H, von Koenig CHW. Bordetella. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 31. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7813/
- Melvin, J., Scheller, E., Miller, J. et al. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 12, 274–288 (2014). https://doi.org/10.1038/nrmicro3235
- Finger H, von Koenig CHW. Bordetella. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 31. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7813/
- Nieves DJ.Heininger U.2016.Bordetella pertussis. Microbiol Spectr4:10.1128/microbiolspec.ei10-0008-2015.https://doi.org/10.1128/microbiolspec.ei10-0008-2015
- Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol. 2014 Apr;12(4):274-88. doi: 10.1038/nrmicro3235. Epub 2014 Mar 10. PMID: 24608338; PMCID: PMC4205565.
- Bart MJHarris SR, Advani A, Arakawa Y, Bottero D, Bouchez VCassiday PK, Chiang C, Dalby T, Fry NK, Gaillard ME, van Gent M, Guiso NHallander HO, Harvill ET, He Q, van der Heide HGJ, Heuvelman K, Hozbor DF, Kamachi K, Karataev GI, Lan R, Lutyńska A, Maharjan RP, Mertsola J, Miyamura T, Octavia S, Preston A, Quail MA, Sintchenko VStefanelli P, Tondella ML, Tsang RSW, Xu Y, Yao S, Zhang S, Parkhill J, Mooi FR2014.Global Population Structure and Evolution of Bordetella pertussis and Their Relationship with Vaccination. mBio5:10.1128/mbio.01074-14.https://doi.org/10.1128/mbio.01074-14
- https://www.ecdc.europa.eu/en/pertussis/facts
- https://www.cdc.gov/pertussis/about/index.html
- https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/bordetella-pertussis
- https://emedicine.medscape.com/article/967268-overview?form=fpf
- https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/bordetella-pertussis.html
- https://www.who.int/publications/i/item/laboratory-manual-for-the-diagnosis-of-whooping-cough-caused-by-bordetella-pertussis-bordetella-parapertussis.-update-2014
- https://www.cdc.gov/pertussis/index.html
- https://www.mayoclinic.org/diseases-conditions/whooping-cough/symptoms-causes/syc-20378973
- https://www.who.int/health-topics/pertussis#tab=tab_1
- https://my.clevelandclinic.org/health/diseases/15661-whooping-cough-pertussis
- https://en.wikipedia.org/wiki/Bordetella_pertussis
- https://universe84a.com/bordetella/