What is Biogeochemical Cycle?
- A biogeochemical cycle, also known as a nutrient cycle, refers to the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth’s crust. It is a cyclical process where nutrients and elements are recycled through the biotic (living) and abiotic (non-living) components of an ecosystem.
- The term “biogeochemical” is derived from the combination of “bio” (biosphere), “geo” (geological components), and “chemical” (elements). It signifies the complex interaction between biological, geological, and chemical processes that contribute to the cycling of nutrients.
- In ecosystems, energy flow is linear, meaning it moves in a single direction from the primary producers to the consumers and eventually dissipates as heat. However, nutrients follow a cyclic pathway. Nutrients are acquired by green plants from the soil through their roots, passed on to herbivores and then to carnivores as organisms consume each other, and ultimately return to the soil through the decomposition of dead remains by detritivores and decomposers. This recycling of nutrients ensures their availability for sustaining life in the ecosystem.
- The Earth’s matter is conserved and exists in the form of atoms. Since matter cannot be created or destroyed, it is continually recycled within the Earth’s system. The major elements involved in biogeochemical cycles include carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. These elements move between the biotic components (biosphere) and the abiotic components, such as the atmosphere, hydrosphere (water bodies), and lithosphere (Earth’s crust).
- There are several major biogeochemical cycles, including the carbon cycle, nitrogen cycle, and water cycle. In the carbon cycle, plants absorb atmospheric carbon dioxide through photosynthesis, converting it into organic compounds. These compounds are then utilized by organisms for energy and growth. Carbon is released back into the atmosphere through processes like respiration and decomposition, as well as through human activities such as burning fossil fuels.
- The nitrogen cycle involves the conversion of atmospheric nitrogen gas into usable forms like ammonia and nitrates through nitrogen fixation, primarily performed by certain bacteria. These compounds are essential for the growth of plants and other organisms. Nitrogen is returned to the atmosphere through denitrification and other processes.
- The water cycle, also known as the hydrological cycle, involves the movement of water between the atmosphere, land, and oceans. It includes processes like evaporation, condensation, precipitation, and runoff. Water is vital for sustaining life and plays a crucial role in various biogeochemical cycles.
- These cycles are interconnected and rely on the interaction between biological, geological, and chemical processes. Microorganisms, such as bacteria, are significant drivers of biogeochemical cycling as they perform essential metabolic processes involved in nutrient cycling. Without microorganisms, many of these processes would not occur, greatly impacting the functioning of ecosystems and the planet’s overall biogeochemical cycles.
- Human activities can disrupt these cycles, leading to environmental problems such as climate change and pollution. Activities like burning fossil fuels and excessive use of fertilizers can contribute to imbalances in the cycles, emphasizing the importance of sustainable practices.
- In summary, biogeochemical cycles are vital processes that involve the movement and transformation of nutrients and elements between living organisms and their environment. These cycles ensure the availability and recycling of essential elements, sustaining life in ecosystems and maintaining the balance of the Earth’s systems.
Definition of Biogeochemical Cycle
A biogeochemical cycle is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth’s crust, involving the cycling and recycling of nutrients and elements essential for life.
Types of Biogeochemical Cycles
Biogeochemical cycles are classified into two main types: gaseous cycles and sedimentary cycles. These cycles involve the movement and transformation of different elements and compounds essential for life on Earth.
- Gaseous Cycles: The gaseous cycles involve elements that primarily exist in gaseous form or are involved in the gaseous phase of the cycle. The main gaseous cycles are:
- Carbon Cycle: The carbon cycle involves the cycling of carbon between the atmosphere, biosphere (living organisms), hydrosphere (water bodies), and lithosphere (Earth’s crust). Carbon dioxide (CO2) is absorbed by plants through photosynthesis, converted into organic compounds, and passed on to other organisms through the food chain. Carbon is released back into the atmosphere through respiration, decay, and combustion of fossil fuels.
- Oxygen Cycle: The oxygen cycle is closely interconnected with the carbon cycle. It involves the movement of oxygen between the atmosphere and the biosphere. Oxygen is produced by plants during photosynthesis and is utilized by organisms for respiration. It is also released back into the atmosphere through the decay of organic matter.
- Nitrogen Cycle: The nitrogen cycle is responsible for the conversion and cycling of nitrogen between various forms. Atmospheric nitrogen (N2) is converted into usable forms like ammonia and nitrates through processes like nitrogen fixation. Plants and other organisms incorporate nitrogen compounds into their tissues, and nitrogen is returned to the atmosphere through processes such as denitrification and ammonification.
- Water Cycle: The water cycle, also known as the hydrological cycle, involves the continuous movement and transformation of water between the atmosphere, land, and oceans. It includes processes like evaporation, condensation, precipitation, and runoff. The water cycle is crucial for the distribution of water resources on Earth and plays a vital role in various ecosystems.
- Sedimentary Cycles: The sedimentary cycles involve elements that primarily cycle through the lithosphere, or the Earth’s crust. The main sedimentary cycles are:
- Sulphur Cycle: The sulphur cycle involves the movement of sulphur between the lithosphere, biosphere, hydrosphere, and atmosphere. Sulphur is released into the atmosphere through volcanic activity and industrial processes. It undergoes transformations in the atmosphere, is deposited onto land and water bodies through precipitation, and is incorporated into organisms through various biochemical processes. Sulphur is also released back into the atmosphere through decay and volcanic emissions.
- Phosphorus Cycle: The phosphorus cycle involves the movement of phosphorus between the lithosphere, biosphere, and hydrosphere. Phosphorus is primarily present in the form of phosphate minerals in rocks and sediments. It is released through weathering and erosion, entering water bodies where it is utilized by plants and other organisms. Phosphorus is then transferred through the food chain and returned to the lithosphere through sedimentation and geological processes.
- Rock Cycle: The rock cycle involves the continuous transformation of rocks through various geological processes. It includes processes like weathering, erosion, deposition, compaction, and lithification. Different types of rocks, such as igneous, sedimentary, and metamorphic rocks, undergo changes over time due to the influence of heat, pressure, and weathering. The rock cycle plays a significant role in the cycling of minerals and elements present in the Earth’s crust.
These gaseous and sedimentary cycles represent the major pathways through which elements and compounds are cycled and recycled in ecosystems. They are interconnected and essential for maintaining the balance of nutrients and sustaining life on Earth.
Components of Biogeochemical Cycles
Biogeochemical cycles consist of two essential components: the reservoir pool and the cycling pool. These components play a crucial role in the movement and storage of nutrients within the cycles.
1. Reservoir Pool
- The reservoir pool refers to the large storage or reservoir of nutrients within the biogeochemical cycle. It can be in the form of the atmosphere or the Earth’s rocks and minerals. These reservoirs hold vast quantities of nutrients that can be released or absorbed by living organisms as part of the cycle.
- For example, in the carbon cycle, the atmosphere serves as the reservoir pool for carbon dioxide (CO2), which is a greenhouse gas. The oceans and fossil fuel deposits in rocks also act as significant carbon reservoirs. These reservoirs store immense amounts of carbon that can be exchanged with other components of the cycle.
- Similarly, in the nitrogen cycle, the atmosphere is the primary reservoir pool for nitrogen gas (N2). Nitrogen is abundant in the atmosphere, but its inert form makes it inaccessible to most organisms. However, certain specialized bacteria can convert atmospheric nitrogen into forms that can be utilized by plants and other organisms.
- The reservoir pool acts as a source or sink for nutrients, supplying or absorbing them as needed by the cycling pool. The movement of nutrients between the reservoir pool and the cycling pool is facilitated by various processes, such as biological, chemical, and geological transformations.
2. Cycling Pool or Compartments
- The cycling pool, also known as compartments of the cycle, refers to the relatively short-term storages of nutrients within the biogeochemical cycle. These compartments include living organisms, such as plants and animals, where nutrients are actively cycled and exchanged.
- In the carbon cycle, for instance, plants serve as a cycling pool as they absorb atmospheric carbon dioxide through photosynthesis, converting it into organic compounds. These organic compounds are then consumed by herbivores, which become part of the cycling pool. The carbon continues to cycle as the herbivores are consumed by carnivores or decompose after death, releasing carbon back into the environment.
- The cycling pool represents the active exchange of nutrients between organisms and their environment. It includes all living components of the ecosystem involved in the uptake, utilization, release, and recycling of nutrients. These processes drive the movement of nutrients through the biogeochemical cycles, ensuring their availability for sustaining life.
- It’s important to note that the cycling pool and the reservoir pool are interconnected. Nutrients are continually exchanged between these pools, with the cycling pool drawing upon the reservoir pool for nutrients and returning them back to the reservoir pool through various biological, chemical, and geological processes.
Overall, the components of biogeochemical cycles, namely the reservoir pool and the cycling pool, work together to facilitate the movement, storage, and exchange of nutrients within ecosystems. The cycling pool represents the active cycling of nutrients among living organisms, while the reservoir pool serves as a larger storage of nutrients in the atmosphere or rocks, ready to be utilized by the cycling pool.
Examples of Biogeochemical Cycles
1. Carbon cycle
The carbon cycle is a crucial natural process that involves the exchange of carbon between the atmosphere, oceans, land, and living organisms. Carbon, in the form of carbon dioxide (CO2), is the primary source of carbon for this cycle. Let’s explore the different steps involved in the global carbon cycle:
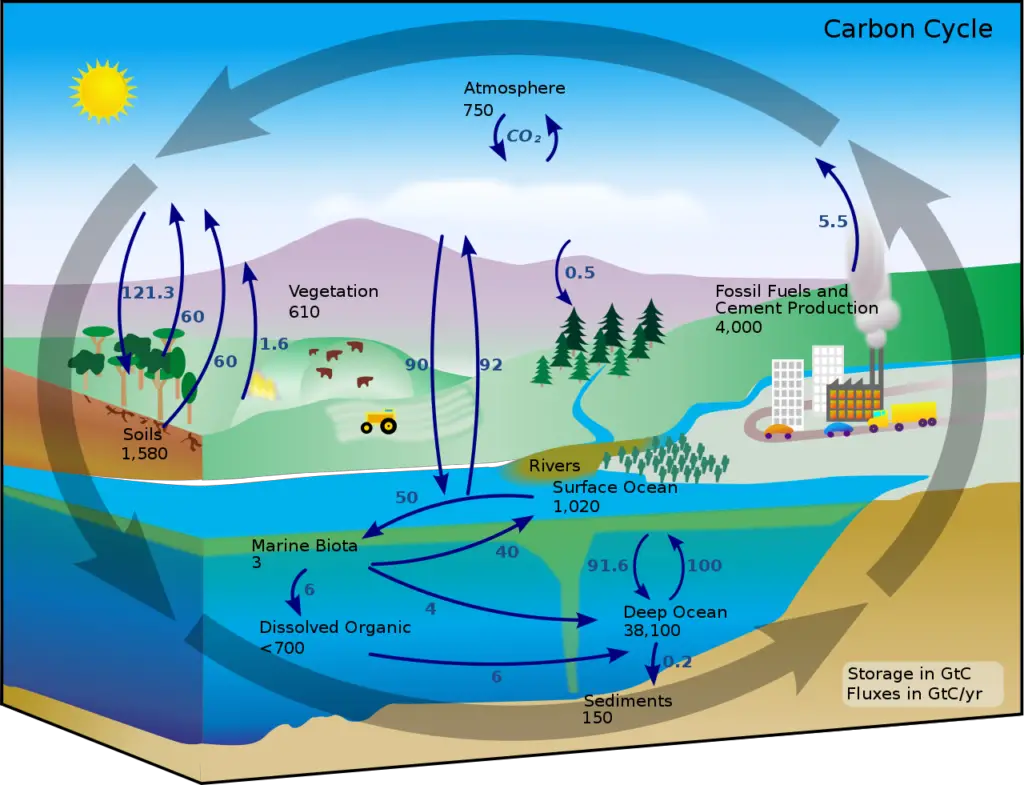
- Photosynthesis: One of the most important processes in the carbon cycle is photosynthesis, which is carried out by green plants and some microorganisms. In the presence of sunlight, plants absorb carbon dioxide from the atmosphere and use it, along with water, to produce glucose and oxygen. This organic matter serves as food for plants and is stored as their biomass. Photosynthesis is responsible for fixing a significant amount of carbon annually, ranging from 4 to 9 x 10^13 kilograms.
- Respiration: All living organisms, including plants, animals, and decomposers, engage in respiration. During respiration, organic matter (food) is broken down to release energy, carbon dioxide, and water. This process occurs in cells and is vital for sustaining life. As a result of respiration, carbon dioxide is released back into the atmosphere.
- Decomposition: When living organisms die, their bodies or plant materials become a source of organic matter for decomposers such as bacteria and fungi. These decomposers break down the dead organic matter and release carbon dioxide into the atmosphere as a byproduct. Decomposition plays a crucial role in recycling nutrients and returning carbon back to the environment.
- Combustion: Burning biomass, such as forests or fossil fuels, also releases carbon dioxide into the atmosphere. Forest fires contribute significantly to carbon dioxide emissions through combustion. Additionally, human activities like burning of fossil fuels for energy production and transportation release substantial amounts of carbon dioxide into the atmosphere.
- Impact of Human Activities: Human activities, especially since the advent of the industrial era, have had a significant impact on the carbon cycle. Large-scale deforestation and the use of fossil fuels in industries, power plants, and automobiles have led to an increased emission of carbon dioxide. These activities have disrupted the natural balance of the carbon cycle and contributed to the rising concentration of CO2 in the atmosphere, leading to global warming.
The disruption of the carbon cycle due to human activities has far-reaching consequences. The accumulation of carbon dioxide and other greenhouse gases in the atmosphere contributes to the greenhouse effect, trapping heat and causing global temperatures to rise. This phenomenon, known as global warming, has adverse effects on climate patterns, ecosystems, and the overall balance of our planet.
2. Nitrogen cycle
The nitrogen cycle is a vital process that involves the transformation and recycling of nitrogen in various forms to support the growth and survival of living organisms. Although nitrogen makes up a significant portion of our atmosphere, it cannot be directly utilized by most organisms. Let’s explore the key processes involved in the nitrogen cycle:
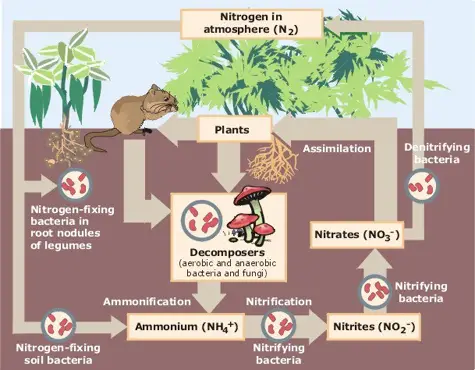
- Nitrogen Fixation: Nitrogen fixation is the process of converting gaseous nitrogen from the atmosphere into a usable form such as ammonia (NH3) that can be utilized by plants. There are three primary methods of nitrogen fixation:
- Atmospheric Fixation: Certain natural phenomena such as lightning, combustion, and volcanic activity provide the energy required to convert atmospheric nitrogen into ammonia.
- Industrial Fixation: In industrial processes, high temperatures and pressures are used to break down molecular nitrogen, which then combines with hydrogen to form ammonia.
- Bacterial Fixation: Symbiotic bacteria, such as Rhizobium found in the root nodules of leguminous plants, and free-living bacteria like Nostoc, Azobacter, and Cyanobacteria, have the ability to convert atmospheric or dissolved nitrogen into ammonia through biological processes.
- Nitrification: Nitrification is the process by which ammonia is converted into nitrates (NO3-) or nitrites (NO2-) by specific bacteria. Nitrosomonas and Nitrococcus bacteria convert ammonia to nitrites, while Nitrobacter bacteria convert nitrites to nitrates. Nitrates are a crucial source of nitrogen for plants.
- Assimilation: During assimilation, plants take up nitrates and incorporate nitrogen into organic molecules such as proteins, DNA, and RNA. Animals obtain nitrogen by consuming plants or other animals and assimilate it into their own tissues.
- Ammonification: When living organisms produce nitrogenous waste products like urea and uric acid, as well as when organisms die, the organic nitrogen present in their tissues is converted back into inorganic ammonia through the process of ammonification. Ammonifying bacteria play a crucial role in this process.
- Denitrification: Denitrification is the conversion of nitrates back into gaseous nitrogen, completing the nitrogen cycle. Denitrifying bacteria, which thrive in oxygen-depleted environments such as deep soil near the water table, break down nitrates and release nitrogen gas back into the atmosphere. Denitrification is essentially the reverse of nitrogen fixation.
The nitrogen cycle is essential for maintaining the balance of nitrogen in ecosystems. It ensures that nitrogen, a vital component of proteins and DNA, is continuously available for plants, animals, and microorganisms. Human activities, such as excessive use of nitrogen-based fertilizers and the burning of fossil fuels, have disrupted the natural nitrogen cycle, leading to environmental issues like water pollution and the degradation of ecosystems.
3. Water Cycle
The water cycle, also known as the hydrological cycle, is a continuous process that involves the movement and transformation of water on Earth. Water, being essential for life, plays a vital role in shaping our planet’s ecosystems. Let’s delve into the key components and processes of the water cycle:
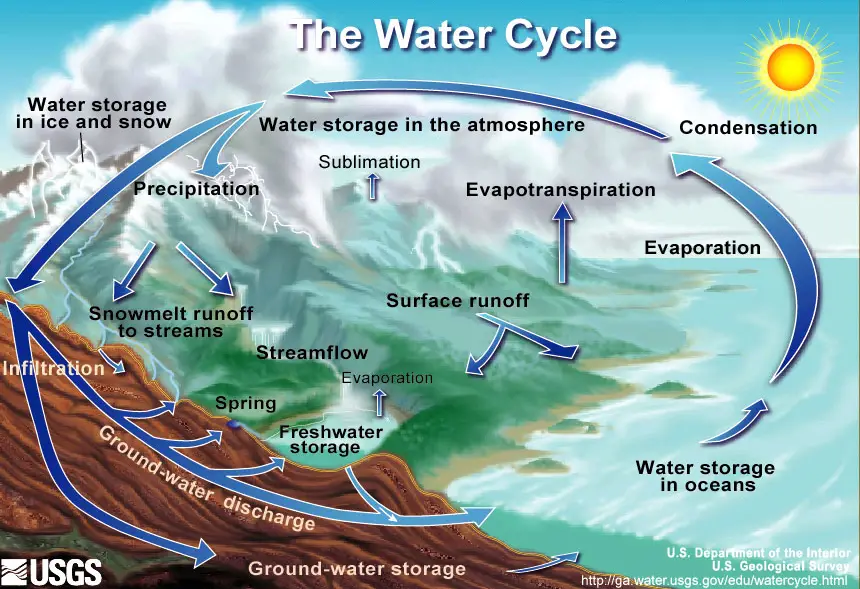
- Precipitation: The water cycle begins with precipitation, which includes various forms such as rain, snow, sleet, or dew. Water is constantly being replenished on Earth through precipitation, which occurs when water vapor in the atmosphere condenses and falls to the surface due to gravity.
- Evaporation: The process of evaporation is a major driving force of the water cycle. Sun’s heat energy causes water bodies such as oceans, lakes, ponds, rivers, and streams to evaporate. Additionally, plants release water vapor through transpiration. The evaporated water rises into the atmosphere as water vapor.
- Condensation: As the water vapor rises higher into the atmosphere, it cools down and condenses to form clouds. Clouds are composed of tiny water droplets or ice crystals suspended in the air. Condensation occurs when the air becomes saturated with water vapor.
- Runoff: When precipitation falls on land, it can either be absorbed into the soil, infiltrating the ground and becoming groundwater, or it can flow over the surface as runoff. Runoff collects in rivers, streams, and eventually makes its way back to the oceans. Runoff plays a crucial role in balancing the evaporation deficit of the ocean.
- Transpiration: Transpiration is the process by which plants release water vapor into the atmosphere through their leaves. It is a significant contributor to the water cycle, with plants absorbing water from the soil through their roots and releasing it into the air through small openings called stomata on their leaves.
The water cycle is driven by two primary forces: solar radiation and gravity. Solar radiation provides the energy required for evaporation and transpiration, while gravity pulls the condensed water droplets down to Earth as precipitation.
It’s important to note that water is not evenly distributed on Earth’s surface. The majority of the Earth’s water, about 97.3%, is found in the oceans, and approximately 2.1% exists as polar ice caps. Only a small fraction, about 0.6%, is available as freshwater in the form of atmospheric water vapor, groundwater, and surface water bodies like lakes, rivers, and streams.
The water cycle is a continuous and dynamic process that helps distribute water resources across different regions and maintain the overall balance of water on Earth. Understanding the water cycle is crucial for managing water resources, predicting weather patterns, and sustaining life on our planet.
4. Oxygen cycle
The oxygen cycle is a biogeochemical cycle that involves the movement of oxygen atoms through various spheres of the Earth, including the atmosphere, biosphere, and lithosphere. It encompasses the production and consumption of oxygen in different forms and highlights its significance in sustaining life. Let’s explore the key aspects of the oxygen cycle:
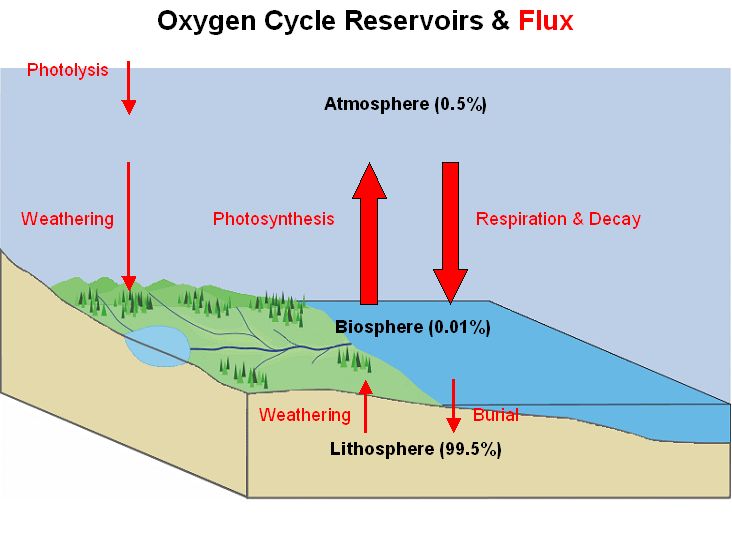
- Oxygen Reservoirs: Oxygen is abundantly present on Earth, with the largest reservoir found in the silicate and oxide minerals of the crust and mantle. These minerals hold approximately 99.5% of Earth’s oxygen. In contrast, the atmosphere, hydrosphere (water bodies), and biosphere (living organisms) collectively contain less than 0.05% of the planet’s total oxygen mass.
- Oxygen in Different Forms: Oxygen exists in various molecular forms within the surface reservoirs of the Earth. The most common form is elemental oxygen, known as diatomic oxygen (O2), which is a product or reactant in numerous biogeochemical redox reactions within the oxygen cycle. Additionally, oxygen atoms are present in molecules such as biomass, water (H2O), carbon dioxide (CO2), nitric acid (HNO3), nitrogen monoxide (NO), nitrogen dioxide (NO2), carbon monoxide (CO), hydrogen peroxide (H2O2), ozone (O3), sulfur dioxide (SO2), sulfuric acid (H2SO4), magnesium oxide (MgO), calcium oxide (CaO), aluminum oxide (AlO), silicon dioxide (SiO2), and phosphate (PO4).
- Biological and Geological Processes: The oxygen cycle involves both biological and geological processes. Biological processes include photosynthesis, which occurs in plants and some bacteria, where they convert carbon dioxide and water into organic compounds, releasing oxygen as a byproduct. This oxygen is then utilized by organisms for respiration, where they consume oxygen and release carbon dioxide.
- Source and Sink: Within the oxygen cycle, processes are categorized as either a source or a sink of oxygen. Sources refer to the production or release of oxygen, while sinks represent the consumption or utilization of oxygen. Photosynthesis acts as a significant source of oxygen, replenishing it in the atmosphere. On the other hand, respiration, combustion, and oxidation processes act as sinks, consuming oxygen from the atmosphere.
The oxygen cycle is closely linked with other biogeochemical cycles, such as the carbon cycle and nitrogen cycle. Photosynthesis, which generates oxygen, is a crucial component of the oxygen and carbon cycles, as it utilizes carbon dioxide and releases oxygen. Additionally, oxygen plays a role in the nitrogen cycle during denitrification, where oxygen is consumed by certain bacteria in the process of converting nitrates back into gaseous nitrogen.
Understanding the oxygen cycle is essential for comprehending the interconnections between Earth’s systems and the maintenance of atmospheric composition necessary for supporting life. It highlights the importance of oxygen as a vital element in sustaining ecosystems and the delicate balance required for its continuous availability.
5. The Phosphorus Cycle
The phosphorus cycle describes the movement of phosphorus through various components of the Earth, including the lithosphere (rocks and minerals), hydrosphere (water bodies), and biosphere (living organisms). Phosphorus is an essential nutrient for living processes, playing a crucial role in DNA, RNA, and energy storage compounds.
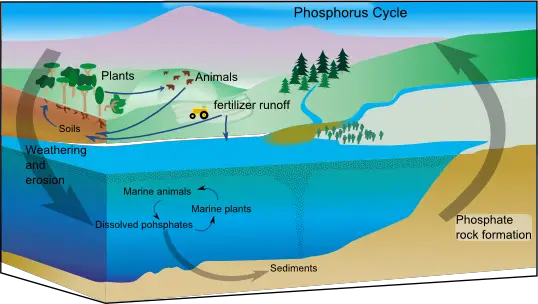
- Phosphate Formation: Phosphorus exists in nature primarily as the phosphate ion (PO43-). Phosphates are released into the environment through natural processes such as weathering of phosphate-containing rocks, where phosphorus is leached out and carried into rivers, lakes, and eventually the ocean. These rocks originate from ocean sediments formed by the remains and excretions of marine organisms. Volcanic ash, aerosols, and mineral dust also contribute to the phosphate pool.
- Geological Processes: Over geologic timescales, the movement of phosphorus occurs through the uplifting of Earth’s surface, bringing phosphate-containing sediments from the ocean onto land. This process is slow and can take thousands to millions of years.
- Phosphate Exchange: Phosphorus is exchanged between the ocean and marine organisms. Marine plants and algae take up phosphate from the water to support growth and metabolism. When these organisms die and sink to the ocean floor, their organic matter becomes part of the sediment, completing the cycle.
- Human Impact: Human activities have a significant impact on the phosphorus cycle. Excessive use of fertilizers in agriculture leads to the runoff of phosphates into water bodies, causing eutrophication. This excess phosphorus stimulates the growth of algae, resulting in algal blooms. As the algae die and decompose, dissolved oxygen is depleted, leading to the formation of dead zones where aquatic life cannot survive.
- Environmental Consequences: Dead zones occur in lakes, rivers, and coastal areas, particularly at the mouths of major rivers. These areas experience a depletion of oxygen due to excessive nutrient inputs, mainly phosphorus and nitrogen. Fish and other aquatic organisms die as a result, disrupting the ecological balance. The Gulf of Mexico, influenced by the Mississippi River’s fertilizer runoff, hosts one of the largest dead zones, spanning thousands of square miles. Other affected ecosystems include the Chesapeake Bay in the eastern United States.
Managing and reducing phosphorus runoff from human activities is crucial to mitigating the negative impacts on aquatic ecosystems. Implementing sustainable agricultural practices, such as precision farming and proper nutrient management, can help minimize phosphorus runoff and maintain a healthy phosphorus cycle. Additionally, raising awareness and adopting responsible wastewater treatment practices can contribute to reducing phosphorus pollution and protecting water resources.
6. The Sulfur Cycle
The sulfur cycle describes the movement of sulfur through various reservoirs on Earth, including the atmosphere, land, and oceans. Sulfur is an essential element for living organisms, playing a crucial role in the formation of proteins and other important molecules.
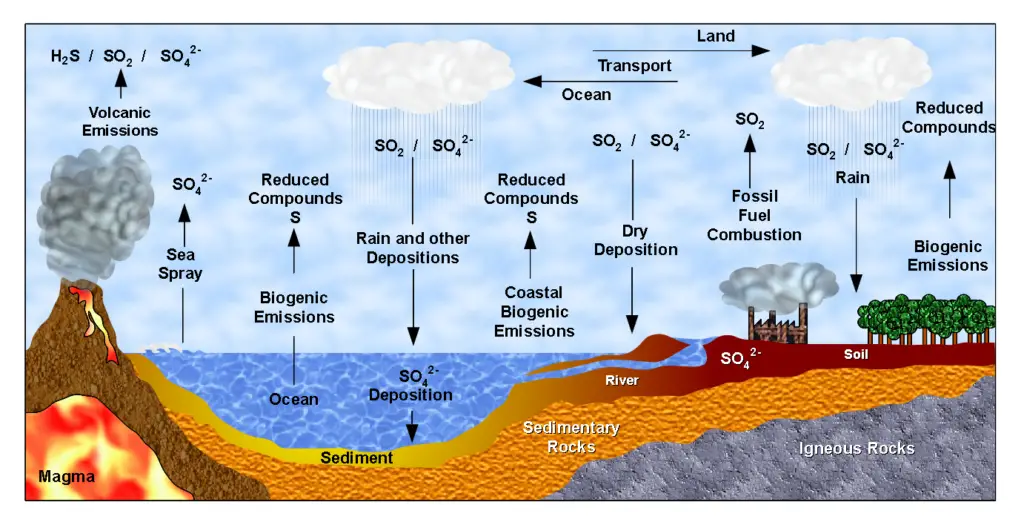
- Atmospheric Sulfur: Sulfur enters the atmosphere in the form of sulfur dioxide (SO2). There are three main sources of atmospheric sulfur: the decomposition of organic matter, volcanic activity, and human activities such as the burning of fossil fuels. Volcanic eruptions and geothermal vents release sulfur dioxide directly into the atmosphere.
- Sulfur Deposition on Land: Sulfur is deposited on land through various processes. Precipitation plays a significant role, as rainwater dissolves atmospheric sulfur dioxide, forming weak sulfuric acid (H2SO4). Sulfur can also directly fall to the ground as fallout from the atmosphere. Additionally, sulfur is released into the soil through the weathering of sulfur-containing rocks, which originated from ocean sediments uplifted onto land over geologic time.
- Sulfur in Terrestrial Ecosystems: Plants take up sulfate ions (SO42-) from the soil through their roots, incorporating sulfur into their tissues. As plants die and decompose, sulfur is released back into the atmosphere as hydrogen sulfide (H2S) gas. Soil microorganisms play a vital role in mediating the cycling of sulfur in terrestrial ecosystems.
- Sulfur in the Oceans: Sulfur enters the oceans through runoff from land, atmospheric fallout, and underwater geothermal vents. Some marine ecosystems rely on chemoautotrophic bacteria that use sulfur as an energy source. Sulfur in the form of sulfates (SO42-) supports the growth of marine organisms and contributes to the marine sulfur cycle.
- Human Impact: Human activities have significantly influenced the sulfur cycle. The burning of large amounts of fossil fuels, particularly coal, releases substantial quantities of sulfur dioxide into the atmosphere. This leads to the formation of acid rain when sulfur dioxide reacts with atmospheric moisture, creating sulfuric acid. Acid rain can have detrimental effects on the environment, including the acidification of lakes and damage to aquatic ecosystems. It also poses risks to human-made structures, causing corrosion and degradation.
Understanding and mitigating the impacts of human activities on the sulfur cycle is crucial for maintaining a healthy environment. Efforts to reduce sulfur emissions, such as the use of cleaner fuels and the implementation of pollution control technologies, are important steps in minimizing the adverse effects of sulfur on ecosystems and human infrastructure.
Factors Influence Biogeochemical Cycles
Biogeochemical cycles are influenced by various factors that can affect the rates and dynamics of nutrient cycling within ecosystems. Some of the key factors that influence biogeochemical cycles include:
- Climate: Climate factors such as temperature, precipitation, and humidity can significantly impact the rates of biogeochemical cycles. For example, temperature affects the rates of decomposition and nutrient mineralization, with higher temperatures generally accelerating these processes. Precipitation patterns can influence nutrient leaching from soils and nutrient runoff into water bodies.
- Soil Characteristics: Soil properties, such as texture, organic matter content, pH, and nutrient availability, influence the cycling of nutrients in terrestrial ecosystems. Soils with high organic matter content generally have higher nutrient retention capacity and support more robust nutrient cycling. Soil pH affects nutrient availability to plants and microbial activity involved in nutrient transformations.
- Vegetation and Land Cover: The type and density of vegetation cover, as well as land use changes, can significantly influence biogeochemical cycles. Different plant species have varying nutrient demands and cycling rates. Deforestation, urbanization, and agricultural practices can alter nutrient cycling by affecting the input and output of nutrients, nutrient uptake by plants, and the microbial activity in soils.
- Human Activities: Human activities have a substantial impact on biogeochemical cycles. Practices such as agriculture, deforestation, industrial activities, and fossil fuel combustion can introduce additional nutrients (e.g., through fertilizer use) or alter natural nutrient cycling patterns. Human-induced changes can lead to nutrient imbalances, increased nutrient losses, pollution, and ecosystem degradation.
- Biotic Interactions: Biotic interactions among organisms, including plants, animals, and microorganisms, can influence nutrient cycling. For example, symbiotic relationships between plants and nitrogen-fixing bacteria enable the conversion of atmospheric nitrogen into a usable form for plants. Predation, herbivory, and decomposition processes also affect nutrient cycling dynamics within ecosystems.
- Hydrological Factors: Water plays a vital role in biogeochemical cycles, particularly in aquatic ecosystems. Hydrological factors such as water flow, availability, and retention influence the transport of nutrients through watersheds, the flushing of nutrients from soils, and the functioning of aquatic biogeochemical cycles.
- Geological Factors: Geological processes, such as weathering, erosion, and volcanic activity, can influence the release and availability of nutrients from rocks and minerals. The geological composition of a region determines the types and quantities of nutrients available for biogeochemical cycling.
- Atmospheric Factors: The composition of the atmosphere, including the concentration of gases and aerosols, can influence biogeochemical cycles. For example, elevated levels of carbon dioxide (CO2) in the atmosphere can affect the carbon cycle and global climate patterns.
It’s important to note that these factors often interact with each other, creating complex feedback loops and responses within biogeochemical cycles. Understanding these factors and their interactions is essential for managing and mitigating the impacts of human activities on nutrient cycling and ecosystem sustainability.
Importance of Biogeochemical Cycles
Biogeochemical cycles play a crucial role in maintaining the balance and sustainability of Earth’s ecosystems. They are of significant importance due to the following reasons:
- Nutrient Cycling: Biogeochemical cycles ensure the continuous recycling and availability of essential nutrients, such as carbon, nitrogen, phosphorus, and sulfur, among others. These nutrients are vital for the growth and functioning of organisms, including plants, animals, and microorganisms. By cycling these nutrients through various reservoirs and compartments, biogeochemical cycles support the productivity and health of ecosystems.
- Energy Flow: Biogeochemical cycles are closely interconnected with the flow of energy through ecosystems. Energy captured from the sun by plants through photosynthesis is transferred to other organisms through food chains and food webs. The cycling of nutrients within biogeochemical cycles provides the energy required for biological processes, such as respiration, metabolism, and growth. Without efficient nutrient cycling, the energy flow within ecosystems would be disrupted, affecting the functioning and productivity of organisms.
- Climate Regulation: Biogeochemical cycles play a vital role in regulating Earth’s climate. For example, the carbon cycle helps regulate the concentration of carbon dioxide (CO2) in the atmosphere, which is a greenhouse gas that contributes to the Earth’s temperature regulation. The cycling of carbon between the atmosphere, plants, and soil helps to mitigate the impacts of climate change by sequestering carbon in vegetation and soils. Additionally, the nitrogen cycle influences greenhouse gas emissions and the formation of ozone, which affects climate patterns and air quality.
- Soil Fertility and Nutrient Availability: Biogeochemical cycles contribute to the maintenance of soil fertility and the availability of nutrients for plant growth. Nutrients released from decomposing organic matter and detritus enrich the soil, providing essential elements for plant uptake. The cycling of nutrients, particularly nitrogen and phosphorus, helps replenish soil nutrient levels, supporting agricultural productivity and the sustainability of natural ecosystems.
- Water Quality and Ecosystem Health: Biogeochemical cycles, such as the nitrogen and phosphorus cycles, impact water quality in aquatic ecosystems. Excessive nutrient inputs, often due to human activities like agriculture and wastewater discharge, can lead to eutrophication, where excessive algal growth depletes oxygen levels in water bodies, harming aquatic organisms. Understanding and managing these cycles are critical for maintaining water quality, protecting biodiversity, and preserving the health of aquatic ecosystems.
- Biodiversity and Ecological Balance: Biogeochemical cycles contribute to the maintenance of biodiversity and ecological balance. The availability of nutrients through these cycles supports the growth and survival of diverse organisms, from microorganisms to plants and animals. The cycling of nutrients and energy within ecosystems ensures the interdependence and functioning of different species, promoting ecological stability and resilience.
In summary, biogeochemical cycles are vital for the functioning, productivity, and sustainability of Earth’s ecosystems. They regulate nutrient availability, energy flow, climate patterns, soil fertility, water quality, and biodiversity. Understanding and managing these cycles are essential for conserving natural resources, mitigating environmental issues, and maintaining the overall health and balance of our planet.
FAQ
What is a biogeochemical cycle?
A biogeochemical cycle is a pathway that describes the movement and transformation of elements and compounds through living organisms, the atmosphere, bodies of water, and the Earth’s crust.
What are the main elements involved in biogeochemical cycles?
The main elements involved in biogeochemical cycles include carbon, nitrogen, oxygen, phosphorus, sulfur, and water.
How do biogeochemical cycles contribute to ecosystem functioning?
Biogeochemical cycles ensure the availability and recycling of essential elements and compounds, allowing organisms to obtain nutrients, energy, and other necessary resources for growth, reproduction, and survival.
What are the key processes in biogeochemical cycles?
The key processes in biogeochemical cycles include the uptake and release of elements by organisms, biological transformations, physical processes like evaporation and precipitation, chemical reactions, and geological processes.
How do human activities impact biogeochemical cycles?
Human activities, such as burning fossil fuels, deforestation, and the use of fertilizers, can disrupt biogeochemical cycles by altering the natural balance of nutrient cycles, contributing to pollution, and causing environmental degradation.
How do biogeochemical cycles interact with climate change?
Biogeochemical cycles, particularly the carbon and nitrogen cycles, are closely linked to climate change. Increased greenhouse gas emissions and altered nitrogen cycling can influence the Earth’s climate, leading to global warming and other climate-related impacts.
What is the role of microorganisms in biogeochemical cycles?
Microorganisms play a crucial role in biogeochemical cycles as they facilitate many of the transformations and processes involved. They participate in nitrogen fixation, decomposition, and nutrient cycling, among other essential functions.
How do biogeochemical cycles affect water quality?
Biogeochemical cycles can impact water quality through processes like nutrient runoff, which can cause eutrophication in water bodies. Excess nutrients, such as nitrogen and phosphorus, can lead to algal blooms and oxygen depletion, negatively affecting aquatic ecosystems.
How long do biogeochemical cycles typically take?
The timeframes of biogeochemical cycles vary depending on the specific element and the processes involved. Some cycles, like the water cycle, can occur relatively quickly, while others, such as the phosphorus cycle, can take thousands to millions of years.
Why are biogeochemical cycles important for sustainable ecosystems?
Biogeochemical cycles are vital for maintaining the balance and sustainability of ecosystems. They regulate nutrient availability, support plant growth, influence climate patterns, and ensure the recycling and reuse of elements, fostering the overall health and functioning of natural systems.
References
- Falkowski, P. G., & Raven, J. A. (2007). Aquatic photosynthesis. Princeton University Press.
- Likens, G. E. (2010). Biogeochemistry of a Forested Ecosystem (3rd ed.). Springer.
- Schlesinger, W. H., & Bernhardt, E. S. (2013). Biogeochemistry: An Analysis of Global Change (3rd ed.). Academic Press.
- Vitousek, P. M., Mooney, H. A., Lubchenco, J., & Melillo, J. M. (1997). Human Domination of Earth’s Ecosystems. Science, 277(5325), 494-499.
- Canfield, D. E., Glazer, A. N., & Falkowski, P. G. (2010). The Evolution and Future of Earth’s Nitrogen Cycle. Science, 330(6001), 192-196.
- Hedin, L. O., Vitousek, P. M., & Matson, P. A. (2003). Nutrient losses over four million years of tropical forest development. Ecology, 84(9), 2231-2255.
- Schlesinger, W. H. (1997). Biogeochemistry: An analysis of global change. Academic Press.
- Holland, H. D. (2006). The oxygenation of the atmosphere and oceans. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1470), 903-915.
- Silver, W. L., Thompson, A. W., & Reich, A. (2005). Ecosystem effects of expanding populations of nitrogen-fixing plants in tropical forests. Annual Review of Ecology, Evolution, and Systematics, 36, 203-229.
- Falkowski, P. G., Scholes, R. J., Boyle, E., Canadell, J., Canfield, D., Elser, J., … & Nakashima, H. (2000). The global carbon cycle: A test of our knowledge of Earth as a system. Science, 290(5490), 291-296.