The Atomic Absorption Spectrophotometer (AAS) is referred to as an analytical instrument that measures the absorption of specific Light wavelength’s by free atom’s, which is required for determining metal concentration in a sample.
It is considered a method where atoms in the Ground State absorbs radiation, leading to characteristic signals that are recorded by the system.
In this technique ,metal element’s are converted into free atom’s, and the absorbed energy is measured, giving rise to quantitative estimation.
- AAS is known as a widely used chemical analysis tool that relies on the interaction between radiant energy and gaseous atom’s , however its operation often involve’s flame / furnace atomization.
- During this process the sample is aspirated into a flame, which is necessary for atom formation, producing measurable absorption lines that indicates concentration.
- The instrument usually contain’s a hollow-cathode lamp (HCL), monochromator, detector and atomizer, and also the Light source is selected so that the emitted line matches the absorption wavelength of the target metal.
- The technique is utilized to detect metals like Fe, Cu, Zn, Pb etc., and sometimes the sensitivity can be influenced by matrix effect’s, giving a look into reaction behavior’s that might be difficult to predict.
- It is characterized by being highly specific, because each element has its own wavelength’s, however—and this is important— interferences can still be observed.
- It is widely regarded as being essential for trace metal analysis in environmental, clinical, agricultural and industrial samples, which leads to important decision-making.
- This technique is applied in water-quality monitoring since metal’s at very low concentration can be detected, resulting in reliable assessment.
- AAS is considered to be a cost-effective instrument compared with ICP-based technologies, though its detection limits for some analyte’s are slightly higher.
- It is associated with regulatory testing (e.g., food contamination, soil evaluation), and also the method can assist laboratories that require routine metal estimation.
- At various phases of analytical work the method is used because it offers good precision ,and the calibration curve’s are usually linear.
- The conceptual foundation is attributed to early 20th-century spectral studies, but the modern form was developed in the 1950s when Alan Walsh proposed the absorption-based quantification approach.
- After this proposal researchers developed hollow-cathode lamp systems, thereby producing instruments that became commercially available around 1954–1955.
- AAS instrument’s were improved across decades, and also new atomization systems (like electrothermal furnace) were introduced, which provides insights into ultra-trace detection capability.
- Over time microprocessor control’s were added, the optical design was refined, and flame / furnace modes were standardized, although some older unit’s kept their mechanical design for long period’s.
- In practice the history of AAS show’s a gradual shift from simple spectral devices to more automated computational instrument’s, forming a sturdy and hardy methodology still used today.
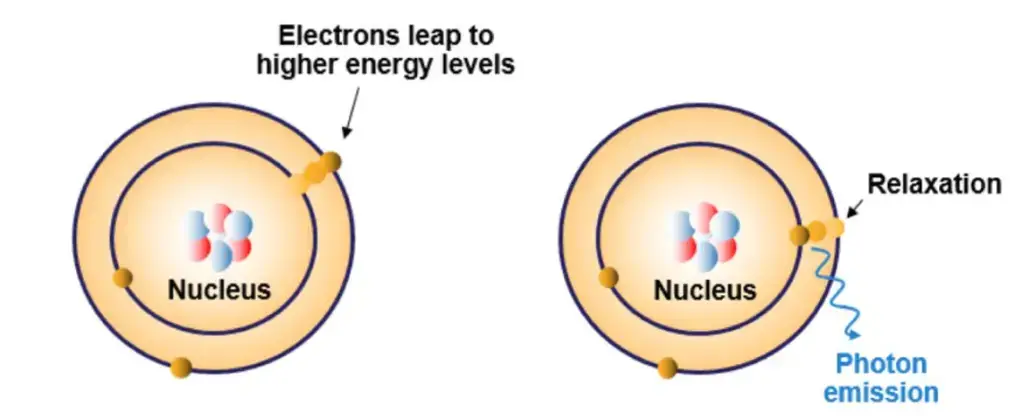
Definition of Atomic Absorption Spectrophotometer
The Atomic Absorption Spectrophotometer (AAS) is a scientific instrument used to quantitatively determine the presence and concentration of specific chemical elements in a sample by measuring the absorbed wavelengths of light by free metallic ions in their gaseous state.
Principle of Atomic Absorption Spectrophotometer
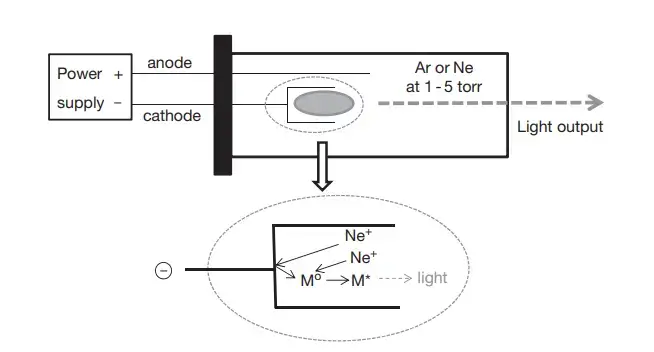
The AAS principle is referred to as the absorption of specific Light wavelength’s by free atom’s in the Ground State, which is necessary for producing a measurable reduction in radiant intensity.
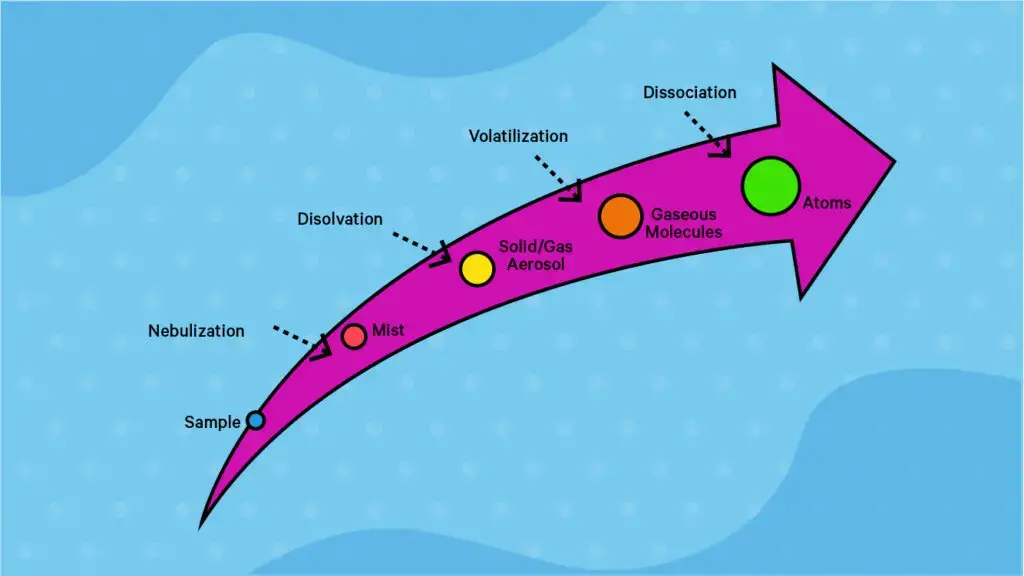
During this process the sample solution is introduced into a flame / furnace atomizer, causing the metal ions to convert in to free neutral atoms, which leads to selective absorption.
The atom’s absorb radiation from a hollow-cathode lamp (HCL) that emits the same characteristic wavelength of the target element, and the reduction in beam intensity is recorded as absorbance.
Absorbance is considered directly proportional to the number of absorbing atom’s in the optical path, although slight matrix effect’s sometimes influence the proportionality.
In this technique the Beer–Lambert relationship is utilized (A=abc), but spacing and Energy fluctuations often prevail the ideal linearity, giving a look into how real analytical systems behave.
The principle is characterized by being element-specific because each atom has its own sharp absorption line’s, however, interference’s from chemical species may be observed, resulting in distorted signals.
Radiation passing through the atom cloud is measured by the detector, which provides insights into concentration, And then converted into electrical output.
At the end of the chain the system calculate’s absorbance by comparing lamp intensity before/after the atomizer, forming the basis of quantitative metal estimation.
The overall principle is recognized as being a “ground-state atom absorption phenomenon”, but human operator’s often describe it more simply as atoms “soaking up” their own wavelength’s.
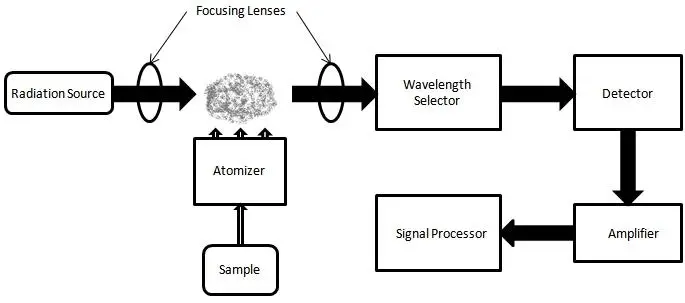
Instrumentation of Atomic Absorption Spectrophotometer (AAS)
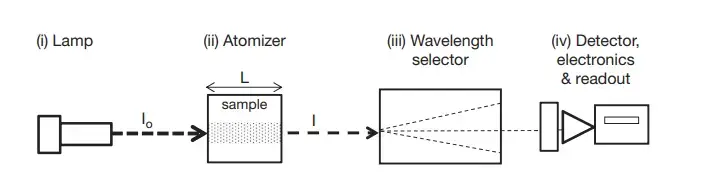
- Light Source
- The Light Source is referred to as the radiation provider emitting very narrow line’s that match the analyte’s wavelength, and Hollow-Cathode Lamp’s (HCL) or EDL unit’s are usually utilized because they generate element-specific emission’s.
- During this process the lamp output is modulated, which is necessary for separating true absorption signal’s from continuous background, also small intensity drift’s may be observed over long runs.
- Atomizer
- The Atomizer is considered the region where sample converting in to free gaseous atom’s occurs, and flame / electrothermal furnace design’s are used depending on sensitivity requirement’s.
- Flame atomizers use chemical combustion Energy to create atom cloud’s, however electrothermal furnace units (like graphite tube) are utilized for ultra-trace work, resulting in higher residence time’s for the atom’s.
- Wavelength Selector
- The Wavelength Selector (mainly the monochromator) isolate’s the desired spectral line, which provides insights into eliminating stray radiation that prevail accurate measurement.
- Echelle-type optics are sometimes applied, giving improved resolution ,and better wavelength focusing, although mechanical alignment drift occasionally influence’s performance.
- Detector
- The Detector is often a PMT (photomultiplier tube), and its output is recorded as changes in light intensity after the atom cloud, producing amplified electrical signals that are converted into absorbance values.
- Modern instruments also use multi-channel detector’s, which can be observed to enhance wavelength coverage and speed, leading to improved analytical throughput.
- Light Modulator
- The Light Modulator is utilized to impose modulation on lamp radiation, so that only the modulated component is measured, thereby producing separation from flame emission ,and reducing noise during analysis.
- Background Corrector
- The Background Corrector is considered vital because it compensates for non-specific absorption and scattering, and methods like Deuterium-lamp correction or Zeeman Effect correction are applied.
- In this case background errors may be reduced significantly, which leads to more reliable quantification especially in samples containing matrix load’s.
- Spectrometer Configuration
- Many AAS units operate in double-beam configuration; the system split’s the radiation into sample path and reference path, resulting in continuous comparison that stabilize’s the instrument output.
- The double-beam design is observed to be advantageous because fluctuations in lamp intensity or optical drift are corrected automatically, also this configuration can assist with long-term signal stability.
- Additional Accessories
- Modern instrument’s include autosampler’s, modular assemblies, direct-solid sampler accessories, and automatic burner-rotation systems, although some laboratory setups utilize only basic modules due to cost.
- These accessories are associated with higher automation, allowing samples to be introduced with less manual intervention, which leads to improved reproducibility.
Types of Atomic Absorption Spectroscopy
1. Flame AAS – Used in many routine metal’s analysis, and the atoms are generated inside a hot Flame that’s usually air–acetylene, which is considered to give moderate sensitivity for element’s detection.
2. Graphite Furnace AAS – also called Electrothermal AAS (ET-AAS)– it is utilized for ultra-trace metals’, because the sample is heated in to a small Graphite Tube, leading to higher Atom population,however, more matrix interference’s can be observed.
3. Hydride–Generation AAS – In this technique volatile Hydride’s (e.g., As,Se,Sn) are produced/ generated chemically, which is needed for improving detection limit of those hydride-forming element’s.
4. Cold Vapor AAS – used mainly for Hg determination, where Mercury vapor is produced at room temperature, resulting in exceptional sensitivity, and also the procedure is simple etc.
5. Continuum Source AAS – a newer approach where a broad-band radiation source is applied, And then high-resolution monochromator separate the specific wavelength’s, giving a look into the entire spectrum/line profile.
Applications of Atomic Absorption Spectrophotometer
- It is utilized for trace-metal quantification in environmental sample’s, like water / soil, giving data that is considered vital for pollution monitoring, And sometimes the detection limit is influenced by matrix load.
- This is applied in clinical/ biomedical labs for metal ions in blood,serum,and urine etc., which is required for diagnosing several metabolic disorder’s.
- They are used in Food & Feed analysis, where elements like Fe,Zn,Cu are measured, leading to assessment of nutritional “Quality”, And also contamination by Pb or Cd is observed.
- It is considered important in pharmaceutical QC, because drug raw-materials are monitored for heavy-metal impurities, which leads to safer formulation’s.
- These are utilized in mining–geology for ore evaluation, the samples are digested then analyzed, producing metal concentration profiles that’s often used for exploration decisions.
- It is applied in industrial process control (e.g. electroplating, alloy making) , and the metal content is measured, however, occasional interference’s may prevail analytical accuracy.
- This can be used in forensic casework, in which element signatures are recorded from residues, resulting in evidential comparison with suspect material’s.
Advantages Atomic Absorption Spectrophotometer
- It is known as a highly sensitive method for metal’s detection, often giving µg/L level readings, which is considered important for trace-analysis.
- This is utilized with relatively simple sample prep, And many matrices are analyzed quickly, giving faster turnaround time than some in-vitro spectroscopic method’s.
- They provide good selectivity for specific element’s, because the wavelength chosen is very narrow, resulting in fewer spectral overlap issue’s.
- It is applied with moderate cost of instrumentation/ operation, although some accessories raise price, the main system still remains accessible for routine labs.
- These are regarded as stable systems, where calibration curves are developed easily, producing reliable quantification even when small fluctuation’s in Flame or Furnace occur.
- It can be operated by technician-level workers, the interface is straightforward, however—And this is important— some advanced furnace steps may require practice.
Limitations Atomic Absorption Spectrophotometer
- It is restricted to single-element measurement per run, which slows multi-element analysis, And sometimes the switching between lines causes extra delay’s.
- This is influenced by chemical / spectral interference’s, especially in complex matrices, leading to signal suppression that is observed during routine water–soil work.
- They require careful lamp selection (HCL/ EDL), and the lamps degrade, producing intensity drift that’s recorded in long analytical sequences.
- It can be limited by relatively narrow linear range, the calibration must be rechecked often, however the drift is not always predictable.
- These are associated with higher maintenance in Graphite Furnace mode, because the Tube is consumed quickly, resulting in recurring cost’s and sometimes inconsistent background correction.
- It is not suitable for many non-metal analyte’s, and the technique mostly focuses on atomic ground-state transitions, giving a look only into specific elemental behavior.
Quiz
What is the primary purpose of an Atomic Absorption Spectrophotometer (AAS)?
a) To measure the concentration of non-metallic elements in a sample.
b) To measure the concentration of metallic elements in a sample.
c) To measure the pH of a sample.
d) To measure the viscosity of a sample.
[expand title=”Show answer” swaptitle=”Hide answer”] b) To measure the concentration of metallic elements in a sample. [/expand]
Which of the following is NOT a type of AAS?
a) Flame AAS
b) Cold vapour AAS
c) Hydride-generating AAS
d) Ultraviolet AAS
[expand title=”Show answer” swaptitle=”Hide answer”] d) Ultraviolet AAS [/expand]
In AAS, what is primarily detected?
a) Atoms
b) Molecules
c) Ions
d) Electrons
[expand title=”Show answer” swaptitle=”Hide answer”] a) Atoms [/expand]
Which of the following gases is commonly used in Flame AAS?
a) Oxygen
b) Nitrogen
c) Acetylene
d) Hydrogen
[expand title=”Show answer” swaptitle=”Hide answer”] c) Acetylene [/expand]
Which component of AAS is responsible for converting the sample into gaseous atoms?
a) Atomizer
b) Detector
c) Monochromator
d) Light source
[expand title=”Show answer” swaptitle=”Hide answer”] a) Atomizer [/expand]
Which of the following is a limitation of AAS?
a) Cannot detect metals
b) Sample is destroyed during analysis
c) High cost per analysis
d) Difficult to operate
[expand title=”Show answer” swaptitle=”Hide answer”] b) Sample is destroyed during analysis [/expand]
In which industry is AAS NOT commonly used?
a) Mining
b) Environmental monitoring
c) Food processing
d) Textile manufacturing
[expand title=”Show answer” swaptitle=”Hide answer”] d) Textile manufacturing [/expand]
What is the role of the light source in AAS?
a) To detect the concentration of the element
b) To convert the sample into gaseous atoms
c) To emit radiation that is absorbed by the sample atoms
d) To separate the wavelengths of light
[expand title=”Show answer” swaptitle=”Hide answer”] c) To emit radiation that is absorbed by the sample atoms [/expand]
Which of the following elements might require a hotter flame in AAS due to its refractory nature?
a) Sodium
b) Potassium
c) Aluminum
d) Lithium
[expand title=”Show answer” swaptitle=”Hide answer”] c) Aluminum [/expand]
Which of the following is an advantage of using Graphite Furnace AAS over Flame AAS?
a) Faster analysis time
b) Suitable for gas samples
c) Requires larger sample volume
d) Higher sensitivity and lower detection limits
[expand title=”Show answer” swaptitle=”Hide answer”] d) Higher sensitivity and lower detection limits [/expand]
FAQ
What is an Atomic Absorption Spectrophotometer (AAS)?
An Atomic Absorption Spectrophotometer (AAS) is an analytical instrument used to measure the concentration of specific metallic elements in a sample by determining the amount of light absorbed by free atoms in a gaseous state.
How does AAS work?
AAS works by introducing a sample into a flame or graphite furnace, where it is atomized. A light source then emits radiation through the atomized sample, and the amount of light absorbed by the sample is measured, indicating the concentration of the element in question.
What types of samples can be analyzed using AAS?
AAS is primarily used for analyzing liquid samples, but with certain attachments, it can also analyze solid samples.
Why is the sample destroyed in AAS?
The sample is atomized, meaning it is converted into free atoms in a gaseous state, in order to measure the absorption of light. This process destroys the original form of the sample.
What are the main types of AAS?
The main types of AAS include Flame AAS (FAAS), Cold Vapour AAS (CV AAS), Hydride-generating AAS (HG AAS), and Graphite Furnace AAS (GF-AAS).
Is AAS suitable for non-metal detection?
No, AAS is specifically designed for the detection and quantification of metallic elements.
How sensitive is AAS?
AAS is highly sensitive and can detect concentrations up to parts per billion (ppb) levels for certain elements.
What gases are used in Flame AAS?
Commonly used gases in Flame AAS include acetylene and nitrous oxide, among others.
Are there any interferences in AAS?
While AAS is mostly free from inter-element interference, certain conditions or elements might cause interference, affecting the accuracy of results.
Can AAS be used for routine analysis in laboratories?
Yes, due to its ease of operation, accuracy, and sensitivity, AAS is widely used in various industries and research laboratories for routine elemental analysis.
- Fernández, B., Lobo, L., & Pereiro, R. (2018). Atomic Absorption Spectrometry: Fundamentals, Instrumentation and Capabilities. Reference Module in Chemistry, Molecular Sciences and
- Chemical Engineering. doi:10.1016/b978-0-12-409547-2.14116-2
Butcher, D. J. (2005). ATOMIC ABSORPTION SPECTROMETRY | Interferences and Background - Correction. Encyclopedia of Analytical Science, 157–163. doi:10.1016/b0-12-369397-7/00025-x
- https://www.agilent.com/en/support/atomic-spectroscopy/atomic-absorption/flame-atomic-absorption-instruments/how-does-aas-work-aas-faqs
- https://www.labcompare.com/Spectroscopy/Atomic-Absorption-Spectrophotometer/
- https://www.technologynetworks.com/analysis/articles/atomic-absorption-spectroscopy-principles-and-applications-356829
- https://lab-training.com/aas/
- https://www.scimed.co.uk/education/what-is-atomic-absorption-spectroscopy-aas/
- https://www.thermofisher.com/in/en/home/industrial/spectroscopy-elemental-isotope-analysis/spectroscopy-elemental-isotope-analysis-learning-center/trace-elemental-analysis-tea-information/atomic-absorption-aa-information.html
- https://www.slideshare.net/sharmasuriti/atomic-absorption-spectroscopy-15185397
- https://saif.iihr.res.in/Instrument/aas-2
- https://www.hitachi-hightech.com/global/en/knowledge/analytical-systems/aas/aas-basics/course3.html