What is Anion Exchange Chromatography?
- Anion exchange chromatography is a powerful technique used in biochemistry and biotechnology for the purification of various substances with negative charges.
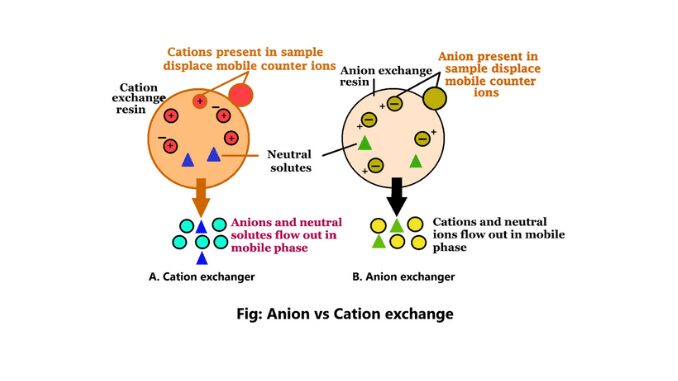
- It is a subset of ion exchange chromatography (IEX), which exploits the differences in net surface charges of molecules to separate them. In the case of anion exchange chromatography, a positively charged ion exchange resin is employed to selectively bind and separate molecules with negative charges.
- The basic principle behind anion exchange chromatography involves the use of an ion exchange resin containing positively charged groups, such as diethyl-aminoethyl groups (DEAE). This resin is typically in the form of small beads or particles.
- In a solution, the resin is initially coated with positively charged counter-ions (cations). When a sample containing the target substances is applied to the resin, the negatively charged molecules present in the sample interact with the positively charged groups on the resin.
- This interaction leads to the displacement of the counter-ions and the binding of the negatively charged molecules to the resin.
- The binding between the negatively charged molecules and the resin is determined by the strength of the negative charge on the molecules.
- Substances with a higher negative charge will bind more tightly to the resin, while those with a lower negative charge will have a weaker interaction and may elute more easily. By controlling the pH of the system, the strength of the negative charge on the target substances can be adjusted, thereby influencing the binding strength to the resin.
- Anion exchange chromatography finds widespread applications in the purification of various biomolecules, including proteins, amino acids, sugars/carbohydrates, and other acidic substances. It is particularly useful for the separation and purification of proteins based on their net surface charge.
- Proteins can carry a net negative charge under certain conditions, such as at higher pH levels. By exploiting this negative charge, anion exchange chromatography allows for the selective separation of different proteins present in a mixture.
- In preparative anion exchange chromatography of proteins, the process is typically performed in a column format. The sample containing the mixture of proteins is loaded onto the column containing the positively charged ion exchange resin.
- The negatively charged proteins in the sample bind to the resin, while unbound molecules are washed away. The bound proteins can then be selectively eluted from the resin by changing the pH or ionic strength of the elution buffer. This elution process enables the separation and collection of individual proteins based on their unique charge properties.
- In addition to preparative purposes, anion exchange chromatography is also utilized for analytical purposes. It enables the characterization and quantification of proteins by monitoring their elution profiles and analyzing the eluted fractions using techniques such as spectrophotometry or mass spectrometry.
- Overall, anion exchange chromatography is a valuable tool in the field of biochemistry and biotechnology for the separation and purification of substances with negative charges. Its versatility and effectiveness make it an essential technique for researchers working in various areas, including protein purification, pharmaceutical development, and biochemical analysis.
Principle of Anion Exchange Chromatography
The principle of anion exchange chromatography is based on the net surface charge of proteins, which changes with the pH and is determined by their isoelectric point (pI). The pI of a protein is the pH at which it carries no net charge. Below the pI, the protein carries a net positive charge, while above the pI, it carries a net negative charge.
By understanding the relationship between pH and the net charge of proteins, researchers can choose an appropriate buffer that ensures a known net charge for the protein of interest. In anion exchange chromatography, a positively charged ion exchange resin is selected when the protein carries a net negative charge at the working pH. The positively charged surface groups on the particles of the anion exchange resin interact with the negatively charged proteins, leading to their binding to the resin.
Proteins with different pI values exhibit varying degrees of charge at a given pH. Consequently, they have different affinities for the positively charged surface groups on the resin particles. This difference in affinity allows for the separation of proteins based on their net surface charge. Proteins with lower pI values will have a stronger interaction with the resin and bind more tightly, while proteins with higher pI values will have a weaker interaction and bind less strongly.
During the chromatographic process, at a specific loading buffer pH, all proteins with the appropriate charge will bind to the resin. For instance, if an anion exchange resin is used at a pH of 7.5, proteins with a pI value lower than 7.5 will carry a net negative charge and will bind to the positively charged resin. To separate the protein of interest from other bound proteins, a salt gradient is employed. The salt concentration is gradually increased, and the proteins are eluted in a specific order based on their net surface charge. Proteins with pI values closer to 7.5 will elute at lower ionic strength (lower salt concentration), while proteins with very low pI values will require a higher salt concentration for elution.
By exploiting the differences in net surface charge of proteins and controlling the pH and ionic strength of the system, anion exchange chromatography enables the selective separation and purification of proteins based on their charge properties. It is a versatile technique used in various applications, such as protein purification, biopharmaceutical development, and protein analysis.
Anion Exchange Chromatography Protocol
Anion exchange chromatography is a powerful technique for separating and purifying proteins based on their net surface charge. While the specific protocol may vary depending on the protein, buffer system, and anion exchange resin being used, the following steps outline a general protocol for anion exchange chromatography:
- Buffer Preparation: Prepare the buffer with the desired pH and ionic strength. It is crucial to titrate the buffer pH after adjusting the salt concentration and ensure that the counterions in the buffer have the same charge as the resin. For positively charged anion exchange resins, Tris buffers are commonly used.
- Column Equilibration: Connect the column to the chromatography system and equilibrate it with the buffer until the pH and conductivity readings stabilize. Typically, this requires passing at least five column volumes of the buffer through the column.
- Sample Loading: Whenever possible, load the protein sample in the starting buffer. The protein’s binding to the anion exchange resin is influenced by the ionic strength and pH, so loading the sample in the appropriate buffer enhances the binding efficiency.
- Column Washing: Wash the column with the loading buffer (0% Buffer B) until no protein is detected in the flowthrough. Typically, washing the column with three to five column volumes of the loading buffer is sufficient.
- Elution: Protein elution can be performed using either a linear gradient elution or a step isocratic elution. Gradient elution is often employed initially to optimize elution conditions and establish the elution profile of the protein. Once the elution conditions are determined, a step elution can be used to speed up the purification process. The elution conditions, such as the ionic strength or pH, should be chosen based on the protein’s elution profile.
- Column Stripping and Equilibration: After the target protein has been eluted, any remaining bound proteins can be stripped from the column resin by increasing the ionic strength or adjusting the pH of the elution buffer. Once all the remaining proteins have been eluted, equilibrate the column in a low ionic strength buffer. If the column will be used in the near future, it is recommended to use the starting buffer for purification. To prevent microbial growth during long-term storage, the column buffer can be exchanged for 20% ethanol in water according to the resin/column manufacturer’s recommendations.
Applications of Anion Exchange Chromatography
Anion exchange chromatography finds a wide range of applications in various fields due to its ability to separate and purify substances based on their net negative charge. Here are some key applications of anion exchange chromatography:
- Protein Separation: Anion exchange chromatography is commonly used for the purification and separation of proteins. Proteins with a net negative charge at the working pH can be efficiently separated using positively charged anion exchange resins. This technique is valuable in protein research, biopharmaceutical production, and other areas where the isolation and purification of specific proteins are required.
- Amino Acid Analysis: Anion exchange chromatography is useful in analyzing and quantifying amino acids. By leveraging the different affinities of amino acids for the anion exchange resin, this technique allows for the separation and identification of individual amino acids within a mixture. It is widely used in protein sequencing, nutrition research, and metabolic studies.
- Nucleic Acid Analysis: Anion exchange chromatography is employed in the separation and purification of negatively charged nucleic acids, such as DNA and RNA. It aids in the isolation of specific nucleic acid fragments, facilitating subsequent analysis techniques like sequencing, polymerase chain reaction (PCR), and genetic engineering.
- Water Purification: Anion exchange chromatography plays a crucial role in water purification processes. It can be utilized to remove harmful anions from water by exchanging them with hydroxyl ions (OH-) present on the anion exchange resin. This technique helps in the removal of contaminants such as nitrates, sulfates, and arsenic ions from drinking water, making it safe for consumption.
- Metal Separation: Anion exchange resins can effectively separate and recover metals from complex mixtures. Metals often form negatively charged complexes that can bind to the anion exchangers. By adjusting the pH and ionic strength, specific metals can be selectively bound to the resin and then eluted separately, allowing for their purification and concentration. This is valuable in various industries, including mining, environmental analysis, and metal recycling.
Advantages of Anion Exchange Chromatography
- Versatility: Anion exchange chromatography can be applied to a wide range of molecules, including proteins, nucleic acids, and other negatively charged compounds. It offers versatility in separating and purifying various analytes.
- High Resolution: Anion exchange chromatography provides high-resolution separation, allowing for the purification of complex mixtures with multiple components. It enables the isolation of target molecules with high purity and selectivity.
- Scalability: Anion exchange chromatography is easily scalable, making it suitable for both laboratory-scale research and large-scale industrial production. The technique can accommodate different sample volumes and flow rates, allowing for efficient purification at various scales.
- Mild Operating Conditions: Anion exchange chromatography can be performed under mild conditions, including ambient temperature and near-neutral pH. This makes it compatible with sensitive molecules and helps preserve their structure and activity.
- Reusability: The anion exchange resin used in chromatography columns can be regenerated and reused multiple times, providing cost-effectiveness and reducing waste generation.
Disadvantages of Anion Exchange Chromatography
- Selectivity Challenges: Anion exchange chromatography may face selectivity challenges when separating closely related molecules with similar net negative charges. Fine-tuning the buffer conditions and resin selection becomes crucial to achieving optimal separation in such cases.
- Limited pH Range: The effectiveness of anion exchange chromatography depends on the pH range applicable to the specific resin chosen. Outside the optimal pH range, the binding affinity between the analyte and the resin may decrease, leading to reduced separation efficiency.
- Ion Exchange Capacity: The ion exchange capacity of the resin may limit the maximum binding capacity for target molecules. This can result in the need for larger columns or multiple purification steps to process high sample volumes or concentrations.
- Salt Interference: High salt concentrations in the sample or buffers can interfere with the separation process, leading to reduced binding efficiency or non-specific interactions with the resin. Proper desalting or buffer exchange steps may be required before or during the chromatographic process.
- Potential Protein Denaturation: In some cases, the adsorption and elution conditions in anion exchange chromatography may cause denaturation or loss of biological activity in delicate proteins. Careful optimization of the buffer conditions and elution strategies is necessary to minimize protein damage.
Examples of Anion exchange chromatography
Anion exchange chromatography is a versatile technique that can be applied to various separation scenarios. Here are two examples highlighting the use of anion exchange chromatography:
- Separation of Nucleic Acids: Anion exchange chromatography can be employed to separate nucleic acids, such as DNA and RNA, from complex mixtures obtained after cell destruction. In this process, the negatively charged nucleic acids bind to the positively charged anion exchange resin, while other components present in the mixture, such as proteins and cellular debris, do not bind and are washed away. By carefully adjusting the buffer conditions, including pH and ionic strength, the nucleic acids can be selectively eluted, resulting in their purification and separation from the initial mixture. This purified nucleic acid fraction can then be further analyzed for various downstream applications, including sequencing, PCR, and genetic engineering.
- Protein Separation from Blood Serum: Anion exchange chromatography is commonly utilized in the purification and separation of proteins from crude mixtures obtained from blood serum. Blood serum contains a complex mixture of proteins, including enzymes, antibodies, and other biomolecules. By using an appropriate anion exchange resin, proteins with a net negative charge at the working pH can selectively bind to the resin, while other components, such as salts and contaminants, are washed away. By adjusting the buffer conditions and applying elution strategies, the bound proteins can be subsequently released from the resin, resulting in their separation and purification. This allows researchers to isolate specific proteins of interest from the blood serum, facilitating further analysis, such as protein characterization, enzymatic activity assays, or therapeutic protein production.
These examples demonstrate the utility of anion exchange chromatography in separating nucleic acids from cell lysates and purifying proteins from complex biological mixtures like blood serum. Anion exchange chromatography’s ability to exploit the electrostatic interactions between the negatively charged molecules and the positively charged anion exchange resin makes it a valuable tool in various biochemical and biotechnological applications.
FAQ
What is anion exchange chromatography?
Anion exchange chromatography is a separation technique that utilizes the electrostatic interactions between negatively charged molecules and positively charged functional groups on an ion exchange resin to separate and purify analytes based on their net surface charge.
What are the main applications of anion exchange chromatography?
Anion exchange chromatography is commonly used for the purification and separation of proteins, nucleic acids, and other negatively charged biomolecules. It is also employed in water purification, metal separation, and various other industries.
How does anion exchange chromatography work?
In anion exchange chromatography, the negatively charged molecules in a sample bind to the positively charged functional groups on the resin, while other components pass through. By adjusting the buffer conditions, the bound molecules can be selectively eluted, resulting in their separation from the sample mixture.
What factors affect the binding and elution of molecules in anion exchange chromatography?
The binding and elution of molecules in anion exchange chromatography are influenced by factors such as pH, ionic strength, buffer composition, resin type, and the net charge of the analyte of interest.
What types of resins are used in anion exchange chromatography?
Anion exchange resins typically contain positively charged functional groups, such as diethyl-aminoethyl groups (DEAE) or quaternary ammonium groups. These functional groups facilitate the binding of negatively charged analytes.
Can anion exchange chromatography be used for both preparative and analytical purposes?
Yes, anion exchange chromatography can be applied for both preparative and analytical purposes. It is used for purifying larger quantities of a target analyte in preparative chromatography, while in analytical chromatography, it helps in characterizing and quantifying the analyte in a sample.
What is the difference between gradient elution and step elution in anion exchange chromatography?
Gradient elution involves changing the buffer composition gradually over time, which creates a linear gradient of ionic strength or pH to elute different analytes sequentially. Step elution, on the other hand, involves changing the buffer composition abruptly, resulting in selective elution of specific analytes based on their binding strengths.
Can anion exchange chromatography be used for the separation of metal ions?
Yes, anion exchange chromatography can be employed for the separation of metal ions. Many metal ions form negatively charged complexes that can bind to the positively charged functional groups on anion exchange resins, allowing for their separation.
What precautions should be taken to avoid protein denaturation during anion exchange chromatography?
To minimize protein denaturation, it is important to optimize the buffer conditions, including pH and ionic strength, to maintain protein stability. Additionally, using mild operating conditions, such as lower temperatures, can help preserve the protein’s native conformation and activity.
How can I determine the optimal conditions for anion exchange chromatography?
Optimizing the conditions for anion exchange chromatography involves conducting preliminary experiments to assess the effects of pH, ionic strength, and other parameters on binding and elution. By systematically varying these conditions, you can identify the optimal set of parameters that yield the desired separation and purification outcomes.