What is Acetic Acid?
- The formula for the organic chemical acetic acid is CH3COOH. A methyl group is connected to a carboxyl functional group in this carboxylic acid.
- The IUPAC systematised name for acetic acid is ethanoic acid, and its chemical formula is C2H2O.
- Vinegar is a solution of acetic acid in water that contains between 5 and 20 percent by volume of ethanoic acid. The acetic acid found in it is responsible for its pungent odour and sour flavour.
- A solution of acetic acid that has not been diluted is frequently referred to as glacial acetic acid. It forms crystals that resemble ice at temperatures below 16.6 degrees Celsius.
- As a polar, protic solvent, it has an extensive range of applications. In the realm of analytical chemistry, glacial acetic acid is commonly used to measure weakly alkaline compounds.
Properties of Acetic acid-CH3COOH
| CH3COOH | Acetic Acid |
| Molecular weight/molar mass of CH3COOH | 60.052 g/mol |
| Density of Acetamide | 1.05 g/cm³ |
| Boiling Point of Acetamide | 118 °C |
| Melting Point of Acetamide | 16.6 °C |
Structure of Acetic acid
- In the solid state of acetic acid, a chain of molecules can be observed in which individual molecules are joined to one another via hydrogen bonds.
- Dimers of ethanoic acid can be found in the vapour phase at temperatures close to 120 °C.
- When ethanoic acid is present in a diluted solution, its dimers can be discovered in the liquid state. Solvents that increase hydrogen bonding have a detrimental effect on these dimers.
- The chemical formula for acetic acid is CH3(C=O)OH or CH3CO2H.
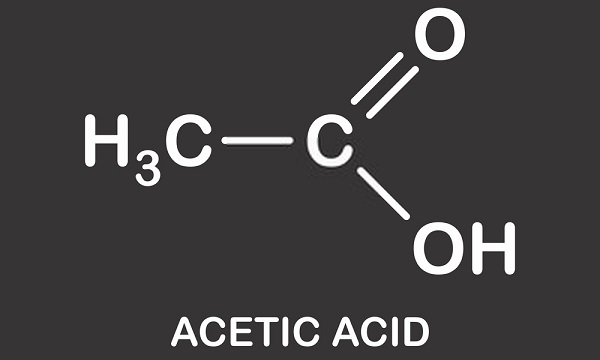
Ethanoic acid is the second-simplest carboxylic acid structurally (the simplest being formic acid, HCOOH), and is essentially a methyl group with an attached carboxyl functional group.
Physical Properties of Acetic Acid
Even though ethanoic acid is considered a weak acid, its concentrated form is highly corrosive and can even attack human skin if it comes into contact with it. Below are some general features of acetic acid.
- Ethanoic acid is a seemingly colourless liquid with a strong odour.
- At standard temperature and pressure, the melting and boiling temperatures of ethanoic acid are 289K and 390K, respectively.
- The molar mass of acetic acid is 60,052 grammes per mole, and its liquid density is 1.049 grammes per cubic centimetre.
- The carboxyl functional group of ethanoic acid can result in ionisation of the molecule, as shown by the following reaction: CH3COOH ⇌ CH3COO– + H+
- The release of the proton, as stated by the equilibrium reaction above, is the underlying source of acetic acid’s acidic nature.
- In a water solution, the acid dissociation constant (pKa) of ethanoic acid is 4.76.
- Acetate is the conjugate base of acetic acid; its formula is CH3COO–.
- The pH of a 1.0M concentration of ethanoic acid is 2.4, indicating that it does not dissociate entirely.
- In its liquid state, acetic acid is a polar, protic, dielectric solvent with a dielectric constant of 6.2%.
The binding of acetic acid to coenzyme A is crucial to the metabolism of carbohydrates and lipids in a variety of organisms. Typically, this chemical is created through the interaction of methanol with carbon monoxide (carbonylation of methanol).
Chemical Properties of Acetic Acid
Similar chemical processes are undergone by acetic acid and other carboxylic acids. This molecule decomposes at temperatures above 440°C to produce either methane and carbon dioxide or water and ethenone, as shown by the following chemical equations.
CH3COOH + Heat → CO2 + CH4
CH3COOH + Heat → H2C=C=O + H2O
Magnesium, zinc, and iron are susceptible to corrosion when exposed to acetic acid. The outcome of these reactions is the creation of acetate salts.
2CH3COOH + Mg → Mg(CH3COO)2 (magnesium acetate) + H2
According to the aforementioned chemical equation, the reaction between ethanoic acid and magnesium results in the creation of magnesium acetate and hydrogen gas.
Other Reactions
As described below, acetic acid interacts with alkalis to generate acetate salts.
CH3COOH + KOH → CH3COOK + H2O
This substance also generates acetate salts by combining with carbonates (along with carbon dioxide and water). Examples of these responses include:
2CH3COOH + Na2CO3 (sodium carbonate) → 2CH3COONa + CO2 + H2O
CH3COOH + NaHCO3 (sodium bicarbonate) → CH3COONa + CO2 + H2O
In the reaction between PCl5 and ethanoic acid, ethanoyl chloride is produced.
Preparation of Acetic acid
Methanol is carbonylated industrially to make acetic acid. Below are the chemical equations for the three phases involved in this process.
- CH3OH (methanol) + HI (hydrogen iodide) → CH3I (methyl iodide intermediate) + H2O
- CH3I + CO (carbon monoxide) → CH3COI (acetyl iodide)
- CH3COI + H2O → CH3COOH (acetic acid) + HI
In this case, the interaction between methanol and hydrogen iodide produces a methyl iodide intermediate. This intermediate is then reacted with carbon monoxide, and the resultant chemical is subsequently processed with water to yield acetic acid. Note that a metal carbonyl complex must be utilised as a catalyst during step 2 of this procedure.
Other Methods of Preparing Acetic Acid
Some cobalt, chromium, and manganese naphthalene salts can be used as metal catalysts in the oxidation of acetaldehyde. This reaction’s chemical equation can be expressed as:
O2 + 2CH3CHO → 2CH3COOH
Using a palladium catalyst and a heteropoly acid, ethylene (C2H4) can be oxidised into acetic acid, as shown in the following chemical reaction.
O2 + C2H4 → CH3COOH
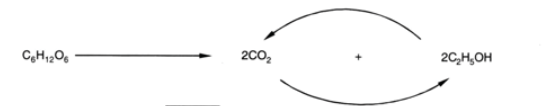
Some anaerobic bacteria are able to convert sugar straight into acetic acid.
C6H12O6 → 3CH3COOH
These bacteria do not produce any ethanol intermediates during the anaerobic fermentation of sugar.
Industrial Production of Acetic Acid
The word vinegar derives from the French word vinaigre (sour wine). In order to produce vinegar, wine must be fermented under certain circumstances. Thus, the creation of vinegar from sugar-containing media involves two stages:
- alcoholic fermentation
- acetic acid fermentation
1. Alcoholic fermentation
- During this phase, sugar-containing substances (such as fruit juices, honey, or hydrolyzed starch) are fermented into ethyl alcohol.
- Yeast enzymes are responsible for this biotransformation (i.e. a selected strain of Saccharomyces cerevisiae).
- One gramme of glucose ought to produce 0.5114 grammes of ethyl alcohol. The fermentable sugar concentration in the raw material undergoing fermentation must be adjusted between 8% and 20%.
- Certain fermentations (such as employing honey as a fermentable substrate) require the addition of potassium and ammonium phosphates to the medium for production.
- In contrast, these mineral salts are not required for fermentations with crushed grapes and grape juice or apple juice. The temperature range of 75 to 80 degrees Fahrenheit has been determined to be optimal.
- In turn, this stage is conducted in two stages:
- Primary stage: Primary phase lasts between 3 and 7 days. Typically, it occurs in open fermenting vats.
- Secondary stage: The duration of the secondary phase is several weeks. It is conducted in sealed fermentors. At the beginning of fermentation, sulphur dioxide is introduced to the production medium prior to the addition of yeast starting culture. This suppresses the growth and activity of yeasts and bacteria that are undesirable. Initial concentrations of 50-100 mg of sulphur dioxide per litre are sufficient.
- Typically, the yield of alcohol is only 85-90 percent of the stoichiometric yield. The clarified wine is extracted from the sediment by racking.
2. Acetic acid fermentation
- There are three primary microbiological mechanisms for the conversion of ethanolic solutions to acetic acid. The following are:
- In the Orleans method, acetic acid bacteria grow slowly as a film on the surface of a still liquid. This is said to make the best vinegar for culinary use.
- The ‘Quick’ vinegar production method that was introduced in the previous century.
- The deep fermentation processes that have only lately achieved commercial success.
- Despite the allure of contemporary deep fermentations, the ‘Quick’ vinegar production method retains considerable industrial significance.
*Quick’ vinegar process
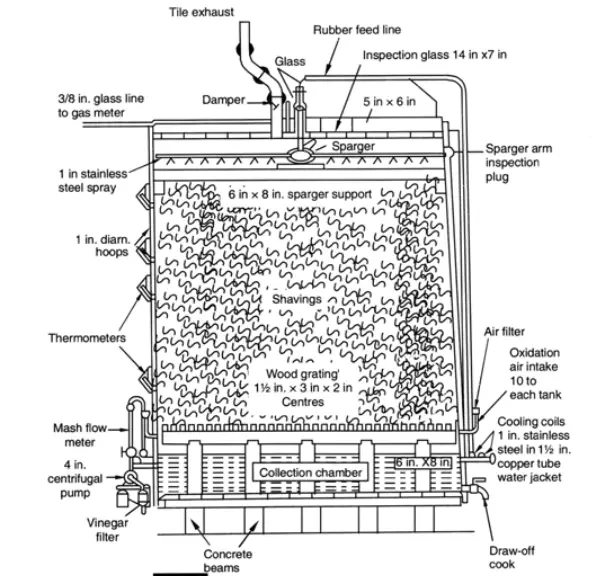
- This method utilises the Fringes generator. It comprises of a tank that is normally 14 feet in diameter and 15 feet in height.
- A wooden grating is located around the base. In addition, the sprayer or distribution arm is installed towards the tank’s top.
- A 4-in vent with a damper coupled to a vapour-liquid separator controls the air supply at the top. Ten air intakes (including air filters) are located near the level of the wooden grate encircling the tank.
- In order to regulate the temperature, the collection chamber at the bottom of the tank is provided with tubular cooling coils.
- The coils are connected to the sprayer’s feed line by a centrifugal pump. The generator is stuffed with beechwood shavings (supported by a wooden grating) to a height of 1 to 5 feet from the top.
- The beechwood shavings are infected with acetic acid bacteria (a chosen species or strain of the genus Acetobacter).
- The vinegar stock or mixture is circulated many times through the beechwood shavings. Consequently, alcoholic solution undergoes increasing oxidation.
- This procedure is repeated until vinegar of the desired concentration is achieved. There are a number of variables that must be regulated during vinegar production. Important considerations for the proper operation of the acetic acid fermentation stage include the type of packing material, the type of vinegar stock utilised, the rate of flow of ethanolic solution, the rate of air flow, and the temperature.
- Generators may be packed with beechwood shavings, coke, pumice, rattan, grape and other twigs, corn cobs, and Berl saddles, among other things.
- For packaging purposes, beechwood shavings are preferable. Allgeier et al. compared the outputs of generators containing beechwood shavings, coke, rattan, and Berl saddles.
- Coke’s limiting factors have been identified as its surface area and impurities. Removing contaminants by acid washing and increasing the surface area by utilising smaller pieces (1/4 to 1/2 in. in diameter) boosted the coke-packed generator’s operating efficiency from 76% to 98% compared to that obtained with costly beechwood shavings.
- Coke packing required a larger concentration and a different sort of nutrient than beechwood packing. Thus, it must be remembered that the type and concentration of nutrients varies depending on the packaging.
- Due to sliming, the use of wine and cider vinegar in recirculating generators is problematic. The growth of the so-called *mother of vinegar* causes sliming (actually exocellular bacterial cellulose produced by Acetobacter xylinum). The production of slime may clog generators of the Frings type.
- Sliming is frequent in generators containing beechwood shavings. In addition, coke-packed generators require repacking after six to twelve months of use.
- In order to prevent clogging, it is also vital to maintain a healthy balance of nutrients. As the concentration of acetic acid in finished vinegar increases, slime formation drops substantially.
- For example, sliming occurs less frequently in cider or wine vinegar stocks that contain sufficient alcohol to create 10 percent vinegar.
- In one experiment utilising the Frings-type pilot plant vinegar generator, Allgeier et al. modified the ethanolic solution flow rate to 12 gallons per hour (100 feet per hour) of packing and the airflow rate to 55 feet per hour (100 ft.3). Given that this is an oxygen-dependent process, it is vital to provide sufficient air:
- C2H5OH +02 – CH3COOH + H2O
- A normal air flow rate is around 80 ft.¾(hr.) (100 ft.3) per hour. Consequently, the airflow rate is adjusted. And the oxygen concentration at the output is kept slightly over 12 percent.
- The optimal operating temperature range for the Frings generator is 80 to 90 degrees Fahrenheit. Since the bioconversion of ethanol to acetic acid is an exothermic reaction, heat is produced.
- Therefore, it is vital to maintain the desired temperature range. Temperatures outside of the optimal range have a negative impact on acetic acid bacteria. In addition, undesirable microorganisms may thrive and produce undesirable chemicals in the finished vinegar.
Deep fermentation process
- This method for acetifying vinegar stock was recently introduced by Hromatka, Ebner, and Haeseler. The “Quick” vinegar production method is currently being replaced by the “Deep fermentation” method.
- They determined that Acetobacter acetigenum and A. pasteurianurn were superior to the other examined species. During their research, fermentors were run at temperatures between 76 and 82 degrees Fahrenheit with 20 litres of air injected every hour.
- The optimal pH range for acetification was between 3.9 and 5.0. When the baseline medium was provided daily to the fermentors, higher quantities of acetic acid were produced than when the medium was continuously diluted.
- Richardson created a method for optimising the production of vinegar from discarded pineapple juice. According to him, a satisfying medium consists of the following elements:
- Corn sugar
- Diammonium hydrogen phosphate
- Autolysed yeast
- Citric acid
- Powdered whey
- Potassium chloride
- In the event of large-scale fermentation, finishing the acetification of vinegar stock is challenging. The fermenting wash must have an alcohol level between 0.25 and 0.30 percent.
- The vinegar produced through submerged fermentation possesses a distinctive flavour and scent.
- It has received positive client feedback. The hazy appearance of vinegar produced by the submerged method is its primary flaw. Therefore, filtering is required to create clear vinegar.
Uses of Acetic Acid
Ethanoic acid is a crucial chemical molecule for human existence. Below are some significant applications of acetic acid.
- Due to its antimicrobial properties, acetic acid is used as an antiseptic.
- Utilizing ethanoic acid in the production of rayon fibre.
- By directly injecting acetic acid into the tumour, acetic acid has been utilised to treat cancer.
- As the primary component of vinegar, it is used to pickle a variety of vegetables.
- Rubber production requires the usage of ethanoic acid. It is also utilised in the production of perfumes.
- It is commonly employed in the manufacture of VAM (vinyl acetate monomer).
- The result of the condensation reaction between two molecules of acetic acid is acetic anhydride.
Acetic Acid as a Solvent
- CH3COOH is a hydrophile (it rapidly dissolves in water) and a polar, protic solvent in its liquid state.
- In this way, a mixture of acetic acid and water is comparable to a mixture of ethanol and water.
- Also miscible with hexane, chloroform, and many oils is acetic acid. It does not, however, create miscible mixes with long-chain alkanes (such as octane).
- Acetic acid is a crucial industrial solvent due to its excellent solvent characteristics and its ability to generate miscible mixes with both polar and non-polar chemicals.
- It is commonly employed in the commercial synthesis of dimethyl terephthalate (DMT).
- In this article, therefore, the general properties, applications, and structure of acetic acid are addressed briefly.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.